The COVID-19 pandemic has been a great challenge for the provision of critical care, especially in patients with severe respiratory failure requiring extracorporeal membrane oxygenation (ECMO) [1]. In order to perform ECMO therapy, constant management of anticoagulation is mandatory, which often results in multiple thrombotic and haemorrhagic events [2, 3]. Unfractionated heparin (UFH) is the most used anticoagulant during ECMO due to its short half-life and the availability of reversal agents [4]. However, the complex pharmacokinetics of UFH require constant monitoring of clotting times during intravenous infusion, and the high risk of life-threatening bleeding may undermine the overall result of the therapy [5].
Advantages of low molecular weight heparin (LMWH) anticoagulation during ECMO include less frequent dosing, decreased activated partial thromboplastin time (aPTT) and potentially lower bleeding risks [6]. According to our previous study on nadroparin administered subcutaneously once daily during ECMO in a non-COVID population, it may serve as a comparable anticoagulant to UFH, especially regarding the influence on the resistance to flow in the oxygenator [7]. According to Lobanov et al. [8], resistance to flow in the ECMO circuit, measured as the difference between pre- and post- oxygenator pressure and divided by flow in the circuit, may serve as a surrogate for the measurement of the risk of thrombotic complications during ECMO therapy. Extended use of nadroparin in the context of ECMO remains understudied, especially in COVID-19 patients. COVID-19 patient care posed unique challenges, as these patients exhibited a heightened prothrombotic state, further complicating anticoagulation management [9, 10]. In the critically ill COVID-19 population, reports have demonstrated that the general thromboembolic risk may reach up to 49% despite thromboprophylaxis [11].
In response to the mentioned observational studies, the strategy of giving the therapeutic dose anticoagulation in critically ill COVID-19 patients has also been tested but did not result in an increased survival rate or cardiovascular and respiratory support- free days compared to regular thromboprophylaxis [12]. While therapeutic anticoagulation has not been proven superior, we have established a pharmaco-kinetic model of low-molecular-weight heparin dosing in different degrees of COVID-19 severity that included patients supported with ECMO [13].
The present study aimed to compare thrombotic and haemorrhagic complications of subcutaneous nadroparin (5700 IU, administered twice daily) and standard UFH anticoagulation during ECMO in critically ill COVID-19 patients.
METHODS
Patient selection and data acquisition
The Ethical Committee of the Medical University of Lublin granted approval for this study (approval number KE-0254/23/2021), as did the Local Ethical Committees for the two ECMO centres involved. Given the study’s retrospective, anonymous, and observational design, it was determined that individual patient consent was not necessary.
This study is a retrospective, bicentric, observational analysis involving critically ill COVID-19 patients who received veno-venous (VV) ECMO support between March 2020 and October 2022. Data collection occurred from October to December 2023, sourcing information from the First Teaching University Hospital in Lublin, Poland, and the University Clinical Hospital in Opole, Poland. Two intensivist specialists determined the initiation of VV ECMO therapy before commencing the treatment, adhering to Extracorporeal Life Support Organisation (ELSO) criteria [14]. These criteria included adult patients with severe acute respiratory distress syndrome (ARDS), a PaO2/FiO2 ratio below 100, unresponsive to standard treatments despite optimized ventilatory settings and prone ventilation, a potentially reversible cause of respiratory failure, and a mechanical ventilation duration not exceeding seven days. The study encompassed 38 consecutive adult patients, of whom 27 were treated with UFH and 11 with a double dose of nadroparin. Patients who received renal replacement therapy were excluded from the study.
UFH and nadroparin dosing
In this study, the group receiving UFH was administered a continuous infusion, with doses adjusted to maintain an aPTT within 60–80 seconds. aPTT was measured four times each day. The group receiving nadroparin was administered 5,700 IU (GlaxoSmithKline Pharmaceuticals, Poznan, Poland) subcutaneously twice daily. In our previous study, we established the population pharmacokinetics of nadroparin to ensure proper low-molecular- weight heparin prophylaxis in the population of critically ill COVID-19 patients [13]. According to the presented model, in patients supported with ECMO, the prophylactic anti-Xa target level of 0.2–0.5 IU mL–1 could be achieved if nadroparin dosing was increased to 5700 IU administered twice daily [13]. Moreover, the effectiveness of nadroparin was monitored by measuring anti-Xa levels four hours following each subcutaneous dose.
ECMO circuit
Patients were connected to the ECMO circuit using two Bioline-coated single-lumen HLS catheters (15–29 Fr) (Maquet Cardiopulmonary GmbH, Rastatt, Germany). The ECMO circuit, composed of standard components with a biocompatible coating from the manufacturer, was connected to a polymethylpentene oxygenator for extracorporeal gas exchange (X Lung Kit, Xenios, Heilbronn, Germany; HLS Set Advanced, Maquet Cardiopulmonary GmbH). A centrifugal pump facilitated blood flow within the circuit. Therapy regulation was conducted using Maquet or ILA Novalung (Xenios AG, Heilbronn, Germany) consoles, ensuring a blood flow range of 3–6 L min–1, as clinically indicated.
ECMO therapy
ECMO parameters, such as the blood flow in the ECMO circuit and sweep gas flow, were fine-tuned to maintain PaO2 levels above 60 mmHg and PCO2 levels below 45 mmHg. Patients underwent protective ventilation, aiming for a 4–6 mL kg–1 tidal volume to achieve either normocapnia or mild hyper capnic acidosis. Sedation was administered, and the use of neuromuscular blocking agents was at the discretion of the attending physician. Temperature control was ensured at a normothermic 37°C using a heat exchanger. Comprehensive care typical of an intensive care unit (ICU) was provided to all patients. This included nutritional support, fluid management, blood products, vasopressors, sedation, and antibiotics as required.
Gradual weaning from ECMO therapy involved reducing the blood flow in the circuit to 3.5 L min–1.
In the group that received subcutaneous nadroparin, the blood flow was maintained at a minimum of 3.5 L min–1 to prevent oxygenator thrombosis. Oxygenator or pump head replacements were not scheduled but were conducted only if defects were observed. Pump replacement was considered when blood flow fell below 2 L min–1 after ruling out issues such as cannula kinking or hypovolaemia. Oxygenator replacement criteria included a drop in the patient’s oxygenation (PaO2 below 60 mmHg) and a post-oxygenation PaO2/FiO2 ratio below 200.
Outcomes
The primary aims of our study were to compare the haemorrhagic and thrombotic complications observed during ECMO therapy. We defined thrombotic complications as instances of acute peripheral thrombosis and variations in resistance to flow in the ECMO oxygenator. The oxygenator was inspected for clot formation by the intensivist four times each day. Additionally, we measured the flow resistance in the oxygenator (R) four times daily, calculated as the pressure difference across the oxygenator divided by the flow rate in the extracorporeal circuit. It has been established that an increase in resistance to flow in the oxygenator is closely linked to thrombosis within the ECMO system [8].
Haemorrhagic complications were categorized based on the frequency of bleeding incidents, including life-threatening ones, the volume of blood products administered, post-ECMO serum haemoglobin levels, and platelet counts during ECMO’s initial seven days. Bleeding assessment was conducted four times daily. Under the ELSO guidelines, life-threatening bleeding is characterized by a drop in haemoglobin levels of at least 2 g dL–1 per day, bleeding over 20 mL kg–1 in 24 hours, or the need for a transfusion of at least 10 mL kg–1 of packed red blood cells (RBC), along with bleeding into areas such as the retroperitoneal space, respiratory system, or central nervous system. Our research focused on comparing the first seven days of ECMO, the median duration of therapy, in patients treated with a single dose of nadroparin. The follow-up period terminated with either the patient’s discharge from the ICU or death.
Statistical analysis
The data’s distribution was examined using the Shapiro-Wilk test. Owing to its non-normal distribution, the data are expressed as medians along with their interquartile ranges. For the comparison of non-parametric continuous data, the Mann-Whitney U test and the Kruskal-Wallis ANOVA were employed. Analysis of proportions and categorical values was conducted using the c2 test. We used linear regression and ordered logistic regression for the comparison between the study groups. All statistical tests were two-sided, with a significance threshold set at P < 0.05. Data compilation was carried out using Microsoft Excel (Redmond, WA), while statistical analyses were performed with Statistica 13.1 software (Stat Soft. Inc, Tulsa, OK, USA) and STATA software (StataCorp, Texas, USA). The ChatGPT 4 tool (OpenAI, San Francisco, USA) was used to assist in editing the discussion section of the present manuscript.
RESULTS
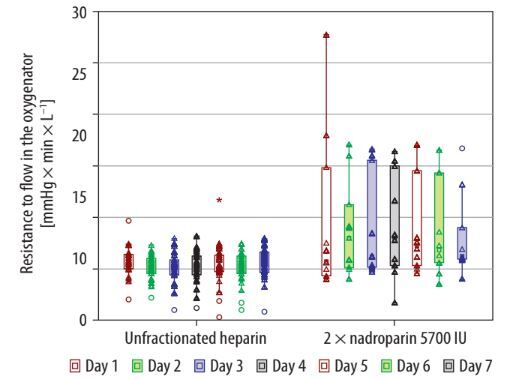
The population of patients included in the study is presented in Table 1. The majority of covariates were equally distributed between the study groups. There was a significant difference in the distribution of sex and ECMO duration between the groups (Table 1). At the end of the study period, we found that the change in resistance to flow in the oxygenator throughout the therapy was significantly smaller in the group anticoagulated with UFH in comparison to nadroparin (1.74% [1.38–2.6] vs. 6.13% [5.93–14.81]; P < 0.001) (Table 2). Moreover, the definite values of resistance to flow in the membrane oxygenator were significantly lower in the group that received intravenously UFH (5.4 mmHg × minute × L–1 vs. 9.4 mmHg × minute × L–1; P = 0.018) (Figure 1). However, in linear regression, we found no differences in the change in resistance to flow throughout the therapy associated with anti coagulation mode (B = 3.74; CI: –4.27 to 11.77; P = 0.349). One case of distal thrombosis was detected in each study group.
TABLE 1
General demographic data and survival
TABLE 2
Outcome comparison between patients receiving nadroparin and unfractionated heparin during ECMO
| Unfractionated heparin i.v. (n = 27) | Twice daily nadroparin 5700 IU s.c. (n = 11) | P-value | |
|---|---|---|---|
| Number of acute thrombotic events, n (%) | 1 (3.7) | 1 (9.1) | 0.5 |
| Change in resistance to flow in the oxygenator during ECMO treatment, % [IQR] | 1.74 [1.38–2.6] | 6.13 [5.93–14.81] | < 0.001* |
| Number of bleeding events, n (%) | 17 (63) | 7 (63.6) | 0.97 |
| Magnitude of bleeding events, n [IQR] | 1 [0–1] | 1 [0–1] | 0.87 |
| Number of life-threatening bleeding events, n (%) | 3 (11.1) | 0 | NA |
| Haemoglobin level after ECMO (g dL–1) | 7.8 (6.9–8.8) | 10.2 (8.5–12.2) | 0.003* |
| Transfused RBC units, n [IQR] | 10 [5–17] | 4 [2–8] | 0.027* |
| Transfused PC units, n [IQR] | 0 [0–1] | 0 [0–0] | 0.22 |
| Transfused FFP units, n [IQR] | 0 [0–0] | 0 [0–3] | 0.33 |
[i] Variables are presented as medians along with their interquartile ranges unless labelled differently.
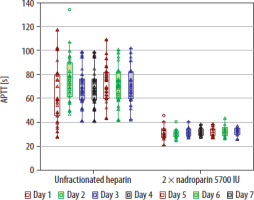
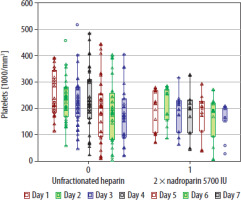
The haemoglobin level after ECMO therapy was significantly lower (7.8 g dL–1 [6.9–8.8] vs. 10.2 g dL–1 [8.5–12.2] ; P = 0.003), and the number of transfused RBC packs was significantly greater in the group that received UFH (10 units [5–17] vs. 4 units [2–8]; P = 0.027) (Table 2). Moreover, nadroparin administration was associated with a significantly decreased number of RBC transfusions (B = –1.35; CI: –2.59 to 0.12; P = 0.031) in ordered logistic regression. Haemoglobin level after ECMO therapy was significantly higher when nadroparin was used (B = 2.39; CI: 1.09–3.69; P = 0.001) in a linear regression model. The length of ECMO therapy was associated with decreased haemoglobin level after ECMO (B = –0.093; CI: –0.167 to –0.021; P = 0.013). However, it was non-significant after adjusting for the choice of anticoagulation (B = –0.053; CI: –0.125 to 0.0198; P = 0.149). The proportion of patients who experienced bleeding complications did not differ between the groups (63% vs. 63.6%; P = 0.97). We found 3 cases of life-threatening bleeding events in the group anticoagulated with UFH and none in the group that received nadroparin (Table 2). aPTT was significantly higher in patients who received UFH (Figure 2). Platelet count did not differ between the study groups throughout the therapy (Figure 3).
Figure 1 illustrates the daily variation in the resistance to flow (R) through the oxygenator during a 7-day ECMO treatment period in patients receiving either UFH or twice-daily nadroparin. This resistance is determined by dividing the pressure differential across the oxygenator by the ECMO circuit’s flow rate, and it is expressed in units of mmHg × minute × L–1 for both patient groups. The median resistance levels are shown as empty squares, while the interquartile ranges are depicted with boxes.
Data for individual patients’ resistance are marked by empty triangles, and outliers are represented by empty circles. A notable finding was that the median resistance to flow in the group treated with UFH was significantly lower than in the group treated with nadroparin over the 7-day ECMO period, as indicated by the P-value of 0.018 in the Kruskal-Wallis ANOVA test.
Figure 2 compares the daily aPTT in patients treated with UFH or nadroparin administered every 12 hours, over a 7-day period of extracorporeal oxygenation. aPTT values are depicted as medians, shown with empty squares, and their interquar-tile ranges are indicated with boxes. Individual patient aPTT results are represented by empty triangles, and outliers are marked with empty circles. The analysis revealed significant differences in the median aPTT values between the two groups during the seven days of ECMO, as indicated by a P-value of 0.001 in the Kruskal-Wallis ANOVA.
Figure 3 depicts a comparison of daily platelet counts in patients undergoing nadroparin treatment either once or twice daily over a period of 7 days of extracorporeal oxygenation. The platelet count values are displayed as medians, indicated by empty squares, along with their interquartile ranges, shown as boxes. Individual patient platelet count data are marked with empty triangles, while outliers are represented by empty circles. The study found no significant difference in the median platelet counts between the two groups throughout the 7-day ECMO period, as demonstrated by a P-value of 0.12 in the Kruskal-Wallis ANOVA.
DISCUSSION
In the present study, we found that the resistance to flow in the oxygenator throughout the ECMO therapy was significantly lower in the group of critically ill COVID-19 patients who received intravenous UFH compared to those who received subcutaneous nadroparin (5700 IU, twice daily). Moreover, the relative change of resistance to flow from the start of the therapy to the seventh day of therapy was significantly smaller in the group that received UFH. At the same time, the number of transfused RBC packs throughout the therapy was significantly higher in the group anticoagulated with UFH, and the haemoglobin level after ECMO therapy was significantly lower in the aforementioned group. The number of life-threatening events was greater in the group that received UFH.
Bleeding and thrombotic events are among the most common adverse events and causes of failure of ECMO therapy [15]. Arnouk et al. [16] reported that bleeding events occurred in 39% of ECMO patients, with a median aPTT of 79 seconds at the time of bleeding. The most severe type of bleeding event during ECMO is intracranial haemorrhage, which occurs in approximately 4.5% of cases [15]. In the present study, there were no life-threatening bleeding events in the group anticoagulated with nadroparin, but there were 3 cases (11.1%) in the group that received UFH (Table 2). The association of aPTT with bleeding in patients undergoing ECMO has been a subject of research, yielding varied findings [15, 17–19]. In the present study, aPTT was significantly different between the study groups (69.27 s vs. 31.5 s). However, the bleeding rate was comparable (63% vs. 63.6%) in the group anticoagulated with UFH and nadroparin, respectively (Table 2). In agreement with the present results, several studies have reported no significant association between aPTT levels and bleeding or thromboembolic events in patients receiving ECMO support. Rajšić et al. [18] and Moussa et al. [19] found no correlation between aPTT values and severe bleeding or thrombotic complications during veno-arterial ECMO support. Furthermore, other authors also concluded that different aPTT-guided protocols do not influence bleeding and thrombotic rate in the population of critically ill patients receiving ECMO [20, 21].
Contrary to the abovementioned rationale, several studies have linked increased bleeding events with increased aPTT. According to Martucci et al. [17], higher aPTT levels may be associated with an increased risk of bleeding during VV ECMO. Furthermore, it is suggested that peak aPTT value may be an independent modifiable factor for bleeding during VV ECMO [22]. Moreover, some authors claim that if bleeding occurs, it may be linked to a higher mortality rate compared to any thrombotic event [15]. Factors that differentially influence the risk of bleeding and thrombosis include patient age, higher body weight, elevated pH at the start of therapy, and a lower ratio of PaO2 to FiO2 [17]. Additionally, Cercone et al. [23] reported that implementing anti coagulation protocols with specific aPTT goals might reduce bleeding in ECMO patients with ARDS. Although the bleeding rate was comparable between the study groups in the present study, the number of RBC transfusions and haemoglobin level after ECMO therapy were significantly smaller in the group anticoagulated with UFH (Table 2). In summary, while some studies suggest a potential association between higher aPTT levels and bleeding risk in ECMO patients, others have found no such correlation, indicating the need for further research to establish a definitive link.
Thrombotic events are the second most feared complication of ECMO therapy. In the population of patients included in the present study, they were comparable between the groups (Table 2). Significant differences in the outcomes representing thrombotic risk were mainly found in the resistance to flow in the membrane oxygenator throughout the therapy, favouring UFH anticoagulation. Our results can be compared to the findings of a study that evaluated the administration of low molecular heparin three times daily. The authors concluded that the implemented anticoagulation protocol during ECMO did not alter the rates of bleeding or thrombosis compared to UFH [24]. Furthermore, Circelli et al. [24] suggested that this revised approach to anticoagulation in ECMO could streamline the management process, lessen the burden on healthcare staff during significant surges of critical respiratory failures in a pandemic, and potentially increase the capacity for ECMO treatment.
Limitations of the present study mainly derive from its retrospective nature and limited sample size. Due to the presented rationale, the study results may be subject to selection bias. Additionally, the possibility of incomplete or missing data, coupled with a small patient sample, may have impacted the comparative outcomes between the groups. In a previous study, we investigated the impact of vasodilators, cardiac output, and kidney function on the pharmacokinetic properties of subcutaneously administered nadroparin, such as absorption, volume of distribution and clearance. We also established a population pharmacokinetic model of its performance in a COVID-19 population supported with VV ECMO. However, the pharmacokinetics of UFH in the population of critically ill COVID-19 patients remains to be established. Specific laboratory data, such as anti-factor Xa and antithrombin III levels, were unavailable due to differing monitoring protocols across centres. Furthermore, factors potentially influencing anticoagulation, such as factor XIII deficiency and acquired von Willebrand syndrome, which could affect bleeding events, were not accounted for. Lastly, the heterogeneity of the COVID-19 population supported with VV ECMO during the pandemic may be subject to other potential confounders that still need to be elucidated.
CONCLUSIONS
Based on the findings of this study, intravenous UFH may be preferable to extended nadroparin administered subcutaneously twice daily for optimizing flow in the membrane oxygenator during ECMO therapy in the group of critically ill COVID-19 patients. However, when UFH is used, the risk of life-threatening bleeding needs to be taken into consideration. Further prospective randomized studies are needed to fully elucidate the presented issue.