The surge in road traffic accidents has led to an increase in orthopedic operations, necessitating effective perioperative pain management [1]. Most orthopedic procedures are performed under subarachnoid block (spinal anesthesia). However, when used alone, 0.5% hyperbaric bupivacaine alone has a short duration of action, often requiring rescue analgesics or conversion to general anesthesia [2]. Adjuvants such opioids (morphine, sufentanil, fentanyl) enhance analgesia but pose side effect concerns [3]. Newer adjuvants (clonidine, ketamine) have potential, but adverse effects limit widespread adoption. Optimal pain control remains a challenge in orthopedic surgery.
The use of dexmedetomidine (DEX) as an adjuvant to spinal anesthesia via various routes such as intra thecal, epidural, intravenous, and intramuscular has already gained popularity. All these routes are invasive, and intravenous bolus dosing of the drug causes acute hemodynamic changes such as brady-cardia, hypotension, and sedation [4].
The intranasal route of drug administration is known for its advantages, including being non- invasive, quick, easy, and well tolerated by patients. Intranasal DEX, in particular, offers good bioavail-ability and a reduced incidence of side effects when compared to intravenous or intra thecal administration [5]. In the literature, most of the stu dies are on intranasal DEX for premedication in pediatric patients. None of the studies evaluated the effect of intranasal DEX on the characteristics of spinal anesthesia in adult patients.
Therefore, the primary objective of this study was to evaluate the effect of preoperatively administered intranasal DEX on the onset, regression, and duration of hyperbaric bupivacaine-induced spinal anesthesia. Secondary objectives included assessing its effects on sedation, hemodynamic parameters, and potential adverse effects.
METHODS
The procedures followed in this study were as per the ethical standards of the Institute Ethics Committee (IEC) and with the Declaration of Helsinki, 2013. The study was conducted after obtaining approval from IEC (2037/IEC-AIIMSRPR/2021 dated 30 November 2021). The study was registered prospectively in the Clinical Trials Registry – India (registration number CTRI/2022/02/040313, www.ctri.nic.in).
It was a prospective, randomized, and double-blind study. Sixty patients, aged 18 to 65 years, of either gender, undergoing elective lower limb surgery under subarachnoid anesthesia belonging to the American Society of Anesthesiologists (ASA) Physical Status 1 or 2 were included in the study. Exclusion criteria included allergy to the drug, coagulopathy, non-compliant patients, patients on beta blockers or anti-hypertensive drugs, recent history of use of other sedatives and anxiolytics, patients on antidepressants, recent history of upper respiratory infection/history of bronchial asthma, presence of apparent nasal mass, hypovolemia, and local infection over the lumbar spine. Informed written consent was taken from patients undergoing elective orthopedic surgery under spinal anesthesia after explaining the study protocol to them. Patients were informed about the visual analogue scale (VAS), which would be used assess pain in the postoperative period (denoting 0 = no pain and 10 = worst imaginable pain). All patients fasted for 8 hours before the scheduled operation and were premedicated with a 0.25 mg tablet of alprazolam and a 40 mg tablet of pantoprazole on the night before the surgery.
The patients were assessed in the preoperative area, which included the surgical and medical history, complete general physical examination, airway examination, and medical investigations such as hemogram, liver, and renal function test. On the day of surgery, in the preoperative area, baseline vitals such as heart rate (HR), O2 saturation (SpO2), and mean arterial pressure (MAP) were monitored.
Patients were randomly assigned to one of the two groups using a computer-generated random number table. After placing the patients in the recumbent position, Group A (n = 30) received 1 µg/kg DEX intranasally, and Group B (n = 30) received intranasal normal saline 20 minutes (min) before the subarachnoid block was administered.
DEX was diluted with normal saline, and both preparations were clear, colorless, and unlabeled, prepared in a 1-ml tuberculin syringe. All these drugs were prepared by the anesthetic technician, who was not blinded to group arrangement and not involved in the administration or data collection of anesthesia for the patients. The drug was prepared based on the random number selected by the patient from the random table and its corresponding group. The group allocation was also concealed by keeping the random number and table centralized with the guide of the study. Double blinding was achieved by concealing the study drug from both the patients and the anesthesiologist administering the sub-arachnoid block, monitoring patients, and recording data.
After noting the baseline vitals in the preoperative area, the study drug was administered to the patients via the intranasal route. The patient was then taken into the operation theatre, and standard ASA monitors were connected. In the operation theatre, baseline vitals were noted and 3 mL of 0.5% hyperbaric bupivacaine was administered via the intrathecal route at the L3–L4 intervertebral space, with the patient in a sitting position.
The recorded parameters included the onset of sensory block (time from injection of local anesthetic in the intrathecal space until the patient began experiencing tingling and numbness), the maximum level of sensory block (the highest level seen in four consecutive pinprick tests), and the two-segment regression time (the time taken for two segment regressions from the highest level of sensory block achieved, assessed every 20 min perioperatively). Duration of postoperative analgesia (defined as the time from the spinal injection to the first request for rescue analgesics, or VAS > 4) was recorded.
A modified Bromage scale was used for assessing the onset and the highest grade of motor block achieved. Grade 1 was taken as the onset of motor blockade. The time taken to achieve grade 3 motor block was noted.
The patient’s haemodynamics (HR, MAP, and SpO2) were recorded at 3 and 5 min, followed by measurements every 5 min for 30 min, every 10 min until 60 min, and subsequently, at 15-minute intervals until 180 min and hourly for 6 hours in the postoperative period. Any decrease in the HR and MAP below 20% from the baseline was treated as per institutional protocol.
The patient’s pain was constantly monitored throughout the procedure by assessing two-segment regression time and in the post-operative care unit via the VAS. A VAS score greater than 4 was considered to be significant. The patient’s sedation was monitored every 30 min throughout the intraoperative and postoperative periods by the sedation score using the six-point Ramsay Sedation Scale (RSS).
Rescue analgesia, paracetamol, or opioids (short- acting) were given if the patients complained of pain at the incision site during or after the procedure. Any adverse events before, during, or after the procedure were treated appropriately.
The sample size was determined to be 26 patients per group to detect a hypothesized 25% difference in postoperative analgesia duration, with 80% power and 95% confidence. However, we decided to recruit 30 participants per group.
Statistical analysis
The statistical analysis was conducted using SPSS Version 23 (IBM Corp.). Continuous variables are presented as mean ± SD, median, and interquar-tile range. Categorical variables are expressed as frequencies and percentages. Group comparisons for normally distributed data were made using the independent sample t-test. If the data deviated from normal distribution, appropriate non-parametric tests, such as the Wilcoxon test, were employed. The χ2 test was used for group comparisons of categorical data. Fisher’s exact test was substituted when the expected frequency in contingency tables was less than 5 for more than 20% of the cells. Linear correlation between two continuous variables was examined using Pearson’s correlation for normally distributed data and Spearman’s correlation for non-normally distributed data. The threshold for statistical significance was set at P < 0.05.
Paired analysis for continuous variables employed the paired t-test when comparing two continuous variables. If the data were non-parametric, the Wilcoxon signed rank test was used instead. Repeated measures ANOVA or the Friedman test was applied for comparisons involving more than two continuous variables.
RESULTS
A total of 60 patients were analyzed. The CONSORT flow diagram is shown in Figure 1. The two groups were comparable to each other in age, gender, ASA physical status, body mass index, type of surgery, and duration of surgery (Table 1). The mean duration of surgery in group A was 89.25 ± 4.97 min and 87.50 ± 4.77 min in group B and was not found to be statistically different (P < 0.156). The common procedures performed included open reduction with internal fixation for fractures of the femur and both lower limb bones, knee and ankle surgery, debridement, and implant removal. The distribution of the procedures among the groups was even (contingency coefficient: 0.212, P < 0.78).
TABLE 1
Demographic variables
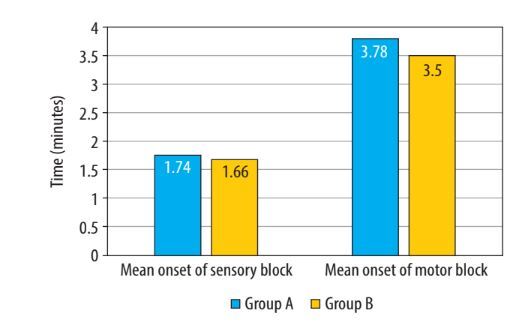
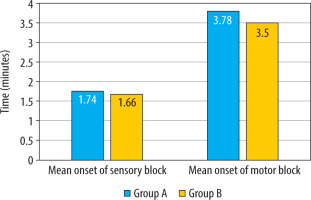
In terms of block characteristics, there was no statistically significant difference between the groups for the onset of sensory and motor blockade (Figure 2, Table 2). There was no significant difference in the distribution of the maximum level of sensory blockade between the 2 groups (χ2 = 2.441, P = 0.279) (Table 3). In the majority of patients, the maximum level of sensory block attained was T8 in both groups. In Group A, 17 (56.7%) patients, and in Group B, 22 (73.3%) patients, achieved T8 sensory block (P = 0.279). However, one patient in group A achieved the highest sensory level of T6.
TABLE 2
Sensory motor parameter following subarachnoid block
TABLE 3
Maximum level of sensory blockade
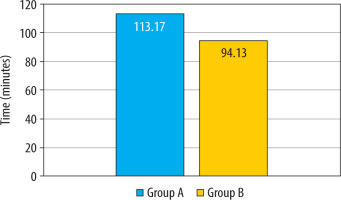
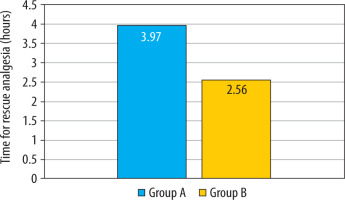
The mean two-segment regression time, as shown in Table 2, was significantly longer in the group that received intranasal DEX in combination with hyperbaric bupivacaine (Group A: 113.17 ± 14.11 min vs. Group B: 94.13 ± 9.59 min; P < 0.001) (Figure 3). The mean time for requesting first rescue analgesia was statistically significantly longer in the intranasal DEX group, providing a longer duration of postoperative analgesia (Group A: 3.97 ± 1.56 h vs. Group B: 2.56 ± 0.76 h; P < 0.001) (Table 2, Figure 4). The mean time for the duration of motor block (P = 0.371) and recovery (P = 1.00) of motor blockade was comparable between the two groups.
There was a gradual and significant fall in the mean heart rate between the two groups for the first 15 min after spinal anesthesia (P < 0.001) (no patient required atropine injection). After this period, the changes in the mean heart rate stabilized and remained comparable between the groups throughout the perioperative period (Figure 5A). The MAP was comparable between the two groups, and neither the average intraoperative MAP (P = 0.335) nor the average postoperative MAP (P = 0.615) showed any discernible changes (Figure 5 B).
FIGURE 5
a) Change in mean heart rate over time compared between groups. b) Change in mean arterial pressure over time compared between groups. SAB – sub-arachnoid block

The mean postoperative VAS score for Group A was 1.97 ± 0.58 and for Group B was 2.49 ± 0.51. There was a significant difference in the average postoperative VAS score between the two groups (t = –3.614 – parametric t-test, P < 0.001), with Group B having the highest mean postoperative VAS score (Figure 6A).
FIGURE 6
a) Change in visual analogue scale (VAS) score over time compared between groups. b) Change in Ramsay Sedation Scale (RSS) score over time compared between groups. SAB – sub-arachnoid block

Although the overall RSS did not differ significantly between the two groups, intraoperatively at 30 min and 60 min, the change in RSS score was found to be statistically significant (P < 0.001) (Figure 6B).
DisCussion
Orthopedic surgery often involves severe postoperative pain, and regional anesthesia offers benefits including better pain control, reduced opioid use, and fewer opioid-related side effects. The goal for perioperative physicians in such procedures is to ensure prolonged analgesia without significant physiological disturbances. Given the shorter duration of action of spinal bupivacaine, our study utilized intranasal DEX as an adjuvant. The results showed that intranasal DEX is a safe and effective non-invasive adjuvant when used with spinal bupivacaine. It improved the duration of the blockade, extended the time to the requirement for postoperative rescue analgesics, maintained stable haemo-dynamics, and was free of adverse effects.
Alpha-2 adrenergic receptor (a2-AR) agonists have recently gained attention for their wide range of effects, including analgesic, perioperative sympatholytic, sedative, anesthetic-sparing, antisialogogue, antishivering, and hemodynamic-stabilizing pro perties [6]. DEX, a highly selective a2-AR agonist with a significantly higher α2/α1 activity ratio (1620 : 1), offers all these benefits without causing respiratory depression. This makes it a safe and valuable adjunct in various clinical settings [7]. Moreover, because of its synergistic effect with local anesthetics, it prolongs the duration of subarachnoid anesthesia [2, 8].
DEX provides analgesia through spinal, supra-spinal, and peripheral mechanisms [9]. It is commonly administered via various invasive routes, including intravenous, intrathecal, epidural, caudal, and intramuscular, as well as non-invasive routes such as buccal and intranasal. Intranasal administration of drugs allows them to cross the blood-brain barrier and directly reach the central nervous system [10]. Moreover, due to the nasal mucosa’s high vascularity, medications quickly enter the systemic circulation, thereby bypassing first-pass metabolism in the liver [11]. In a study with healthy volunteers, an intranasal dose of 84 µg of DEX showed a lag time of 2–3 min, with maximum plasma concentration reached at 38 min and a bioavailability of 82% [12]. Therefore, we chose the intranasal route for its rapid onset, ease of use, and being odorless and painless, without the need for an intravenous line.
This route also ensures good absorption and avoids the high peak plasma levels associated with intravenous administration.
The utility of intranasal DEX has been proven in various medical contexts, serving as a premedication for morbidly obese patients undergoing bariatric surgery [13]. Additionally, it has been utilized to attenuate the hemodynamic response to laryngoscopy and tracheal intubation [14], and as a sedative for children undergoing magnetic resonance imaging and computed tomography scans. Moreover, it has been employed as a premedication in children with burns [15, 16]. We studied its effects on the characteristics of spinal anesthesia. Additio nally, Yuen et al. [17] conducted a randomized comparison of two doses of intranasal DEX (1 μg kg–1 and 2 μg kg–1) as premedication in children. They found that both doses yielded comparable satisfactory sedation levels with no adverse hemodynamic effects in any group. In our study, we chose the lower safe dose of 1 μg kg–1 to prevent untoward side effects.
In our study, the time required for the onset of both sensory and motor blockade was not significantly reduced with the administration of intra nasal DEX. Additionally, we found that the maximum level of sensory block achieved was comparable in both groups. Similarly, in Sharma et al.’s study [18], the administration of DEX either intrathecally or intravenously did not impact the onset of spinal anesthesia with hyperbaric bupivacaine.
In the current study, the mean time for two-segment regression of sensory blockade was significantly prolonged in the intranasal DEX group. Similarly, the duration of postoperative analgesia, measured by the time until the first request for rescue analgesia, was longer at 3.97 ± 1.56 hours in the DEX group compared to 2.56 ± 0.76 hours in the plain spinal bupivacaine group, which was both statistically and clinically significant (P < 0.001). These findings can be attributed to the highly selective nature of intranasal DEX for a2-adrenoreceptors, particularly a2A and a2C. Our study results were consistent with those of Kulkarni et al. [19], who found that intranasal DEX in pediatric patients undergoing caudal epidural anesthesia prolonged the duration of both anesthesia and postoperative analgesia (up to 12.47 ± 2.16 h) compared to bupivacaine alone. Similar outcomes have been observed in studies exploring the use of DEX via intrathecal, intravenous, and intramuscular routes, all of which reported extended anal-gesia when combined with hyperbaric bupivacaine during spinal anesthesia [18, 20, 21]. Furthermore, average postoperative pain scores (VAS score < 4, P < 0.001) were lower in the DEX group, reducing the need for polypharmacy and improving patients’ satisfaction in the postoperative period. Tang et al. [22] also reported lower postoperative VAS scores in adults who received intranasal DEX as premedication for FESS surgery compared to a control group.
In our study, motor blockade was comparable between the two groups, indicating that intranasal DEX did not affect the degree of motor block achieved. In contrast, studies involving DEX admini stered intravenously, intrathecally, and intramuscularly showed a significantly prolonged motor blockade [20, 23].
DEX can cause hemodynamic side effects such as hypotension and bradycardia, which depend on various factors, including dose, administration speed, route, and plasma levels. These side effects are often observed when an initial loading dose of DEX is administered intravenously over a short duration (< 10 min), as revealed by a meta- analysis conducted by Nie et al. [24]. Our study addressed this issue by opting for intranasal administration of DEX. Given that intranasal DEX has a relatively slow onset of action (mean onset time 30–45 min) [25], we administered it 20 min before spinal anesthesia. Consequently, the DEX group exhibited a stable hemodynamic profile. Although there was an initial decline in mean heart rate, it stabilized 15 min after spinal anesthesia and remained steady throughout the surgery. Overall, mean heart rates were comparable between the two groups. Moreover, there was no significant difference in frequency of hypotension between the groups, which aligns with findings from other studies, including a meta-analysis by Abdallah et al. [26] and a study by Sheta et al. [27], who observed no significant hemodynamic disturbances in children receiving intranasal DEX at 1 µg kg–1.
In our study, although the overall RSS did not show a significant difference between the two groups, intraoperatively at 30 min and 60 min, the group receiving intranasal DEX exhibited a significantly lower RSS score (3 or 4) without any respiratory depression, and the subjects were easily arousable. Similarly, in Yuen et al.’s [28] study, significant sedation was achieved with intranasal DEX at 45–60 min, with the effect peaking at 90–105 min.
Choosing a medication requires consideration of both its efficacy and potential side effects. Intranasal DEX, which is colorless and odorless, was well tolerated by patients in our study. No adverse effects such as drug allergies, nasal discomfort, bradycardia, hypotension, nausea, or vomiting were observed following its administration.
liMitations
In our study, intranasal DEX was administered 20 min before spinal anesthesia, while the literature indicates that peak plasma concentration following intranasal DEX is observed at 47 min. Therefore, the onset of sensory and motor blockade was not significantly affected in our study. Further research is needed, considering the administration of intranasal DEX 40–50 min before spinal anesthesia. Total analgesic consumption in 24 hours was not noted. Concerns arise regarding the cost of DEX and its reduced availability in resource-poor settings. Pain is a subjective phenomenon, and its assessment using the visual analog method in the same patient at different times might influence the outcome.
ConClusions
The intranasal administration of DEX at a dose of 1 µg kg–1 before spinal anesthesia has been shown to prolong the effect of subarachnoid anesthesia, extend postoperative analgesia, achieve satis factory sedation levels, maintain stable hemo-dynamic parameters, and exhibit no adverse effects. This suggests that intranasal DEX serves as an effective adjuvant, providing a non-invasive and well-tolerated alternative to other invasive routes.