In the evolving landscape of neurocritical care, the diagnosis and management of cerebral vaso-spasm and delayed cerebral ischemia (DCI) following aneurysmal subarachnoid hemorrhage (aSAH) have undergone significant changes in recent years [1, 2]. aSAH, a severe form of hemorrhagic stroke, constitutes a small percentage of all stroke cases with disproportionately greater rates of morbidity and mortality than ischemic injuries [3]. This condition not only challenges the resilience of patients, leaving a substantial number with long-term disabilities, but also tests the acumen of medical professionals in detecting and managing the subsequent risk of DCI, a pivotal factor in patient outcomes [4–6].
DCI, manifesting primarily as a consequence of cerebral vasospasm alongside other proischemic pathomechanisms, presents a complex syndrome that complicates the recovery trajectory of aSAH survivors. Its onset, usually occurring within two weeks after hemorrhage, is intricately linked to a multitude of factors including microvascular dys-function and cortical-spreading depolarization [7]. Despite advancements in understanding and technology, early and accurate detection of vasospasm and DCI remains a formidable challenge, particularly in patients with compromised consciousness, thereby necessitating a multidisciplinary approach to monitoring and intervention.
In response to these challenges, there has been a notable transition towards integrating a variety of diagnostic modalities, each with its own set of advantages and limitations. Catheter angiography, though remaining the gold standard for diagnosing macrovascular spasms, is increasingly complemented by computed tomography (CT)-angiography (A) and CT-perfusion (P) imaging [8]. These methods offer less invasive alternatives and provide reliable assessments of cerebral blood flow, including micro-circulatory dynamics, thereby holding promise for early DCI detection.
Additionally, transcranial Doppler ultrasonography (TCD) and near-infrared spectroscopy (NIRS) neuromonitoring have emerged as valuable noninvasive tools for monitoring cerebral blood flow dynamics. TCD focuses on flow velocities in the anterior, middle, and posterior cerebral as well as the internal carotid artery (anterior cerebral artery – ACA, middle cerebral artery – MCA, PCA – posterior cerebral artery, internal carotid artery – ICA, respectively), offering insights into the state of macrovascular health, while NIRS neuromonitoring, leveraging principles of optical physics, provides continuous, real-time monitoring of cerebral oxygenation. Despite their potential, the reliability and generalizability of these modalities across different patient populations and settings necessitate further validation [9].
Four-channel NIRS technology exemplifies the ongoing quest for non-invasive and continuous monitoring techniques. By measuring the balance between oxyhemoglobin and deoxyhemoglobin concentrations, NIRS offers insights into cerebral oxygenation status, a crucial parameter in managing patients at risk of DCI. However, the application of NIRS neuromonitoring in clinical practice is hampered by variability in device performance, lack of standardized reference values, and the need for more evidence on its impact on patient outcomes [10, 11].
In the present study, the authors ought to investigate the positive predictive value of NIRS neuromonitoring in patients diagnosed and treated for aSAH, presenting with Glasgow Coma Scale (GCS) < 6. The primary endpoint of the examination was the evaluation of NIRS signal falls preceding the development of vasospasm as detected in digital subtraction angiography (DSA). Additionally, a possible correlation between the detecting optode and the intracerebral artery presenting with vasospasm was examined.
METHODS
Patient selection and exclusion criteria
Examined patients were admitted at the authors’ institution between June 2020 and November 2023 and received treatment adhering to the recently published guidelines [1]. Noninvasive neuromonitoring was conducted through daily TCD assessments (examined intracranial arteries were the C7 segment of the ICA, M1 and M2, A1 and A2, P1 and P2 as well as the Lindegaard ratio) and continuous, four-second registrations, four-optode NIRS. Additionally, CT-P was routinely performed between the fifth and the seventh day, and again on the fourteenth day after the onset of symptoms or earlier if neuromonitoring suggested the presence of vasospasm. When relevant flow alterations were detected in CT-P, DSA was performed, with administration of intra-arterial nimodipine if indicated. Patients not presenting vasospasms during their stay, or not surviving the first line of treatment for the ruptured aneurysm (endovascular or surgical), were excluded from the analysis. As the present study reports on findings obtained through standard medical management, ethical approval was waived.
NIRS technical features
A Nonin SenSmart Model X-100 Universal Oximetry System was used for the present investigation. This model is a versatile, advanced monitoring device designed for precise and continuous measurement of oxygen saturation levels. It supports multiple oximetry technologies, including regional oximetry (rSO2) and pulse oximetry (SpO2). The four optodes were placed on the cleaned forehead of the patients at the time of admission to the ICU. The cumulative data obtained through the sensors were downloaded and collected on a Windows laptop after the discharge or death of the patient.
Population baseline characteristics
Collected baseline data included age at presentation, sex, GCS, Hunt and Hess, and World Federation of Neurological Surgeons (WFNS) scales [12]. Of note, the scores registered for each scale were those obtained at first neurological assessment; therefore, patients initially observed at an external institution and presenting with mild clinical symptoms, eventually worsening during the transfer to the authors’ institution, maintained the initially received scores. This choice was made to avoid a systematic “delayed presentation” bias. Furthermore, the modified Fisher scale (mFisher) was used to assess the spread of SAH in an axial CT scan. Aneurysm localization was defined using CT angiography (CT-A) and subsequently confirmed with DSA. The date of endovascular or surgical therapy was also registered. NIRS neuromonitoring and TCD vasospasm screening were started on the first postoperative day.
Literature search strategy and selection criteria
A comprehensive, systematic literature search was performed in compliance with the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [13]. The investigation of articles was conducted through electronic databases, including MEDLINE/PubMed, EMBASE, PLoS, and Cochrane Library. The scope of inclusion encompassed case reports published between January 1997 and January 2024. The primary research terms (“delayed cerebral ischemia”) AND (“subarachnoid hemorrhage”) AND (“near-infrared spectroscopy”) were employed in various MeSH combinations in the titles and abstracts of articles. Inclusion criteria comprised case reports, case series, and original articles detailing at least one patient with SAH caused by aneurysmatic rupture, assessing the role of NIRS neuromonitoring in the prediction of clinically relevant vasospasm. Citations were screened for duplicates, and references within the examined manuscripts were scrutinized. Articles deemed irrelevant, including research, review articles, meeting abstracts/summaries, edito rials, and studies lacking pertinent neuromonitoring and radiological data, were excluded.
Two authors independently reviewed all abstracts to identify articles that required full-text review. Abstracts were screened against the predefined eligible criteria, and all included studies were discussed with a third author. Each publication was thoroughly examined to critically report salient aspects and limitations of NIRS-based neuromonitoring in aSAH patients.
Statistical analysis
Descriptive analysis of the data was performed using the mean, median, and maximum and minimum values. Continuous variables were expressed as mean and range values unless otherwise specified. Normality assessment of numeric variables was performed using the Kolmogorov-Smirnov test. Correlation analysis between variables was executed by calculating the Pearson coefficient for normally distributed variables or the Kendall coefficient for non-Gaussian distributions. Data processing and analysis were performed using SPSS version 24.0.1.1 (IBM, Armonk, NY) and Microsoft Excel. Statistical significance was established for a p < 0.05. Additionally, Cohen’s D coefficient was computed to examine the effect size of the sample, and these findings were interpreted in accordance with the expanded test interpretation proposed by Sawilowski [14].
RESULTS
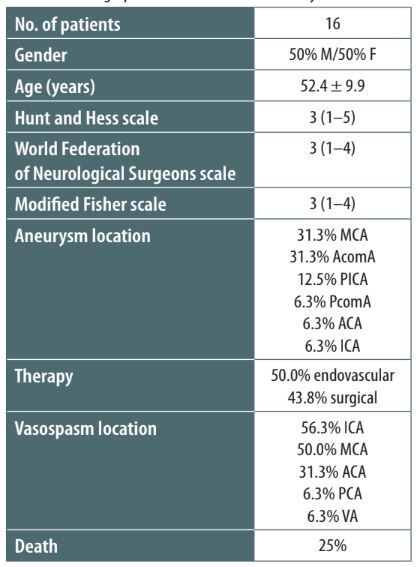
Sixteen patients were included in the final examination, ranging in age from 30 to 64 years, with an average age of 52.4 years. The demographic composition was evenly split between genders. The initial assessment of patients using the Hunt and Hess and WFNS scoring systems yielded a median score of 3, whereas the median score on the modified Fisher scale was recorded as 3 (Table 1). Predominant aneurysm locations were identified at the anterior communicating artery (AcomA) and the MCA, each accounting for 31.3% of cases (80% of MCA aneurysms were located at the bifurcation). A significant proportion of aneurysms were located within the anterior cerebral circulation, which includes the ICA, ACA, AcomA, and MCA. An exceptional case was represented by a patient presenting with perimesencephalic SAH exhibiting no detectable aneurysm rupture upon examination through CT angiography, MR angiography, or two instances of DSA, the latter conducted after the reabsorption of SAH. Surgical intervention through open clipping was the chosen course of treatment for 43.8% of the participants, whereas half of the cohort received endovascular treatment.
TABLE 1
Demographic characteristics of the study cohort
Vasospasms occurring within the ICA were identified as the most prevalent, affecting 56.3% of the patients, followed by vasospasms within the MCA (50%). Bilateral spasms were observed in 31.3% of the study group, with 60% of these being associated with AcomA aneurysms. Signs of vasospasms were detected through routine CT-P in 75% of cases and during TCD examinations in the remaining cases. Alterations in TCD readings were recorded in 68.8% of patients, with 62.5% being detected before the first diagnosis of vasospasms in DSA. Interestingly, in all the cases in which TCD anomalies were detected before the onset of vasospasms, the MCA presented flow alterations in DSA. NIRS measurements were found to be altered in 43.8% of the cohort, with 25% of these alterations occurring before the onset of vasospasms. Finally, the prognosis for the cohort was predominantly unfavorable. The death rate was 25%, and the average modified Rankin Scale (mRS) score at discharge from the ICU was reported as 5, indicating severe disability.
TABLE 2
Results of univariate analysis comparing near-infrared spectroscopy (NIRS) alteration before and after the onset of major vasospasm
An evaluation of the collected parameters failed to establish a statistically significant correlation between age and early pathological values recorded by a 4-channel NIRS device (P = 0.948). Although more males were present in the subgroup of patients with premature NIRS alterations, this did not reach statistical significance (P = 0.200). Additionally, clinical characteristics of aSAH, including Hunt and Hess, modified Fisher, and WFNS scores, did not demonstrate a significant correlation with the alterations observed in NIRS measurements, nor did the location of the bleeding aneurysm.
A focused analysis of individual vasospasm locations revealed a correlation between the presence of alterations in the ACA territory and early alterations in NIRS measurements (P = 0.046; sk = 0.533). Notably, 75% of patients with ACA vasospasms exhibited early NIRS measurement alterations, compared to 18.2% of patients without ACA spasms. Further statistical evaluation yielded a Cohen’s D value of 0.579 (95% confidence interval: 0.102–1.186), which is considered to represent a medium effect size according to Sawilowski’s expanded interpretation, suggesting a moderate practical significance of this correlation. It is of interest that almost two-thirds of patients who experienced a decrease in NIRS signals eventually developed vasospasms in the ACA territory, although this observation did not achieve statistical significance (P = 0.379). No statistically significant difference in clinical outcomes was observed between patients exhibiting NIRS alterations preceding confirmed vasospasms and those without pre-vasospasm NIRS manifestation or developing them after positive DSA (P = 0.936 for mortality; P = 0.626 for mRS).
Value of NIRS neuromonitoring in aSAH
One of the first clinical experiences investigating the role of NIRS-based neuromonitoring for the prediction of DCI in aSAH was reported by Budohoski et al. in 2012 [15]. In their study, the authors detected a significantly increased risk of developing DCI when early intracerebral autoregulation was impaired within five days following admission to the intensive care unit (ICU). Constructing a multivariate model, they found that both the TCD-derived autoregulation index (Sxa), calculated as the linear correlation coefficient between values of systolic flow velocity and arterial blood pressure, and NIRS-based TOxa, calculated as the linear correlation coefficient between values of tissue oxygen index (TOI) and arterial blood pressure (ABP), were independent predictors of DCI. Interestingly, ABP was used instead of cerebral perfusion pressure (CPP), since these two measures were found to have a good correlation; therefore, referring to ABP would ease the calculation of Sxa and TOxa [16]. In line with these early results, other groups further confirmed the reliability of NIRS measures and derived indexes for the prediction of DCI with a combined predictive value of 100% when integrating TCD and NIRS-derived data [17–20].
In their article, Park et al. [11] reported their experiences of predicting DCI and the presence of vasospasms using NIRS-based neuromonitoring and TCD continuous and intermittent examinations, ultimately comparing the two techniques. Fifty-two patients presenting with aSAH and managed with coil embolization were included in the 14-day analysis. Although no statistical significance in the predictive value yielded by NIRS and TCD monitoring was found, they reported a sensitivity of 94.44% and a specificity of 70.59% for identifying DCI when rSO2 measured with 4-optode NIRS declined by at least 12.7%. The choice to only enroll coiled patients was driven by the several difficulties posed by craniotomy, pneumocephalus, and fluid in patients whose aneurysm was clipped, which ultimately represents a major limitation for the generalizability of the results. Furthermore, DCI was assessed using the following criteria: (1) new neurologic changes, including a decreased GCS score of at least 2 points, and (2) severe cerebral vasospasm diagnosed in DSA. The former criterion represents a further limitation of this as well as other studies, since it precludes the identification of DCI in sedated patients during the NIRS and TCD neuromonitoring phase (i.e. approximately 14 days, as an accepted mean duration of the period where the risk of vasospasm is highest [21]), thus prompting the enrollment of low-grade (HH < 3) rather than poor-grade aSAH in observational studies [22]. To overcome some of these limitations, the same group led by Dr. Jeon investigated the significance and reliability of NIRS neuromonitoring in poor-grade aSAH coiled patients for fourteen days [10]. They ultimately found a significant difference in rSO2 detected with a 2-channel system beginning on day 6 between non-DCI and DCI patients. Interestingly, in this cohort of patients, a decline by more than 14.7% of the rSO2 level indicates a sensitivity of 85.7% and a specificity of 85.7% for identifying DCI.
Keller et al. [23] compared values derived from a standard NIRS neuromonitoring system placed on the patient’s skin with intracerebral NIRS measurements to assess the role of interposed tissues between cutaneous NIRS optodes and brain parenchyma. They injected 0.3 mg kg–1 of indocyanine green through a central venous line and performed simultaneous measurements of NIRS cutaneous optodes and NIRS brain tissue probe (intracranial pressure probe associated with optical fibers) in a patient with poor-grade aSAH. After twelve measurements, they found a significant correlation between calculated cerebral blood flow (CBF) obtained with the intraparenchymal probe and values detected by the two standard NIRS channels, although the values for blood volume and flow significantly differed between intraparenchymal and cutaneous NIRS measurements. To overcome the risk of placing intraparenchymal probes when not strictly needed, Tanaka et al. [24] examined the validity of a 44-channel NIRS monitoring system in 29 aSAH patients. This system may detect parenchymal oxygenation impairments in different regions of the brain, since it is not limited to the forehead as its optodes are also applied to the temporal-parietal skull. For instance, the authors demonstrated how this system was able to construct a topographical map of the oxygenation levels within cerebral arteries, demonstrating a strong correlation between pathological events and onset of DCI.
Finally, Kieninger et al. [25] examined the actual application in the clinical setting of multimodal neuromonitoring through an intraparenchymal oxygen measuring probe, standard NIRS, and intracranial pressure probe in 26 high-grade aSAH patients. They found that less than 50% of cases where pathological events were detected through the multimodal system ultimately led to clinical interventions such as modifications in minute ventilation, escalation of the norepinephrine dosage, or elevation of CPP. These results further demonstrate that the placement of parenchymal probes for invasive neuromonitoring or non-invasive NIRS optodes does not per se positively affect outcome following aSAH, but rather a beneficial effect will be observed if values reflecting a real problem in cerebral oxygen supply are regularly and reliably provided through the probes and if direct action is taken to revert the process of DCI.
DISCUSSION
In the present study, the authors aimed to evaluate the reliability and predictive value of a 4-channel NIRS-based neuromonitoring system in patients with high-grade aSAH developing major cerebral vasospasm. Management of aneurysmatic bleeding was performed within 24 hours after neurological deterioration with either surgical or endovascular treatment and multimodal intracerebral monitoring through TCD, and NIRS was initiated soon after the admission of the patients to the ICU. The primary endpoint was represented by the rate of major cerebral vasospasms confirmed by DSA preceded by a fall in the rSO2 detected by NIRS or TOI revealed by daily TCD examinations. The secondary endpoint was the relationship between the optode detecting the rSO2 drop and the location of the observed vasospasm. Despite failing to demonstrate a significant predicting value, the location of the rSO2 reduction in the four optodes significantly correlated with the location of the spastic artery as confirmed by DSA. This investigation confirms the role of integrated NIRS-based neuromonitoring in the complex armamentarium of intensive care practitioners for the early detection of intracerebral vasospasms and DCI, especially in intubated patients. NIRS neuromonitoring thus represents a valuable adjunct to TCD and imaging with perfusion in clinical settings of high- and low-grade aSAH.
Pathophysiology of DCI
Unlike ischemic stroke, the incidence of aSAH has remained stable in recent years, affecting 9 per 100,000 people in the USA [26]. Globally, the rates are between 11.5 per 100,000 for women and 9.3 per 100,000 for men [27]. Despite representing the less common form of stroke, the mortality of aSAH at 21 days after onset of symptoms is in the range of 25–35% and 40–48% in high-income and low to middle-income countries, respectively [26]. Furthermore, morbidity remains a major issue for these patients and society also due to the greater costs than ischemic stroke owing to the younger age of aSAH patients (75 years vs. 52 years) [28, 29]. Despite advancements in medical care, aSAH remains a major medical challenge due to its complex patho-physiology and the significant potential sequelae. The prognosis of patients with aSAH is strongly influenced by the development of DCI, for which no standard treatment to prevent its onset yet exists. DCI has been defined as a clinically observable neurological deterioration initially believed to be solely caused by the presence of relevant cerebral “clinical” or “angiographic” vasospasm, with the latter being observed in unconscious patients. Nevertheless, this paradigm began to shift in the early 21st century, when it was demonstrated that DCI is caused by multiple processes, including vasospasm in cerebral arteries, ischemia, cortical spreading depolarization, microthromboembolism, loss of autoregulation, and capillary transit time heterogeneity [30]. Despite the recognition of a much more complex pathophysiology of DCI, the timely recognition and management of major vasospasms remain of crucial importance for the neurointensivist.
Benefits of NIRS neuromonitoring for prediction of DCI
Different technological advancements have been recently introduced into clinical practice to predict the onset of vasospasm and optimize its treatment early. Since its first application in the ICU, NIRS-based neuromonitoring has emerged as a significant advancement in the neurocritical care setting, primarily due to its non-invasive nature and the ability to provide real-time information on cerebral oxygenation and hemodynamics [31, 32]. NIRS operates on the principle that near-infrared light can penetrate the skull and measure the absorption by oxygenated and deoxygenated hemoglobin, providing an indirect assessment of CBF and oxygenation [9]. One of the major benefits of NIRS highlighted is its non-invasiveness, making it suitable for continuous monitoring without the risks associated with invasive procedures including intraparenchymal measurements of oxygen saturation. Furthermore, NIRS equipment is relatively portable and easy to use, facilitating bedside monitoring in the ICU, potentially reducing the time to intervention and improving patient outcomes [33].
Limitations of NIRS neuromonitoring
Despite these benefits, the application of NIRS neuromonitoring in predicting DCI is not without limitations. One significant challenge is the variability in signal quality, which can be affected by extracranial contamination, patient movement, and the presence of hair [9]. Furthermore, available NIRS sensors in clinical practice do not reliably gather information on hair; therefore, only the frontal hemispheres are sufficiently exposed. This can often mislead the monitoring, as the most frequent perfusion deficits are usually observed in the parietal water-shed zone. Additionally, a 4-channel NIRS device provides a regional measure of oxygenation, which may not accurately reflect global cerebral oxygenation states or specific areas at risk of ischemia due to its limited penetration depth. This limitation underscores the necessity for combining NIRS with other monitoring modalities to obtain a comprehensive understanding of the cerebral hemodynamic state [34]. Furthermore, the specificity of NIRS in detecting DCI among SAH patients remains a topic of ongoing debate. While our results demonstrate a correlation between reduced rSO2 levels and the development of DCI, similar reductions may be observed in other conditions such as systemic hypoxia or hypoperfusion, potentially leading to false-positive results. Therefore, the interpretation of NIRS data should be contextualized within the broader clinical picture. Moreover, large-scale, multicenter studies are required to standardize NIRS monitoring protocols and establish definitive thresholds for intervention.
CONCLUSIONS
This study evaluates the efficacy and reliability of NIRS-based neuromonitoring for predicting cerebral vasospasm in high-grade aSAH patients. Despite its potential for non-invasive, continuous monitoring of cerebral oxygenation, the results of this analysis reveal that its utility in predicting vasospasm and subsequent DCI in high-grade aSAH patients is limited, without significant predictive value in isolation. Nevertheless, NIRS neuromonitoring may predict the location of the spastic artery as confirmed with DSA. Large-scale, multicenter trials are warranted to establish definitive protocols and thresholds to guide clinical decision-making in real time.