In recent years, minimally invasive techniques utilizing surgical robots have been increasingly used in many areas of medicine. Robot-assisted surgery has all the advantages of laparoscopic surgery, such as small incisions, lower surgical site infection rate, less blood loss, less postoperative pain, shorter hospital stay, and faster postoperative recovery. Additionally, robotic surgery offers advantages over laparoscopy that include reduced unintentional camera movements, filtering out the operator’s hand tremors, as well as increased range of motion and degrees of freedom of surgical tools (robotic instruments have six degrees of freedom, while conventional laparoscopic instruments have four) [1, 2].
In Poland, in 2023, 55% of 9,147 prostate cancer operations, 13% of 6,309 uterine cancer operations, and 7% of 14,203 colon cancer operations were performed with robotic assistance [3].
The growing interest in robot-assisted surgical procedures means that more and more anesthesiologists will face the challenge of safely guiding a patient through anesthesia for robotic surgery. The patient’s safety requires that the anesthesiolo-gist be familiar with the structure of the surgical robot, and the principle of its operation, as well as being aware of the potential risks of robot-assisted procedures.
STRUCTURE OF A SURGICAL ROBOT
The global surgical robot market is dominated by Intuitive Surgical Inc.’s da Vinci system. To date, more than 9,100 robots produced by this company have been installed worldwide. They have been utilized in over 14.2 million operations. In Poland, the da Vinci system is used by 43 hospitals [3].
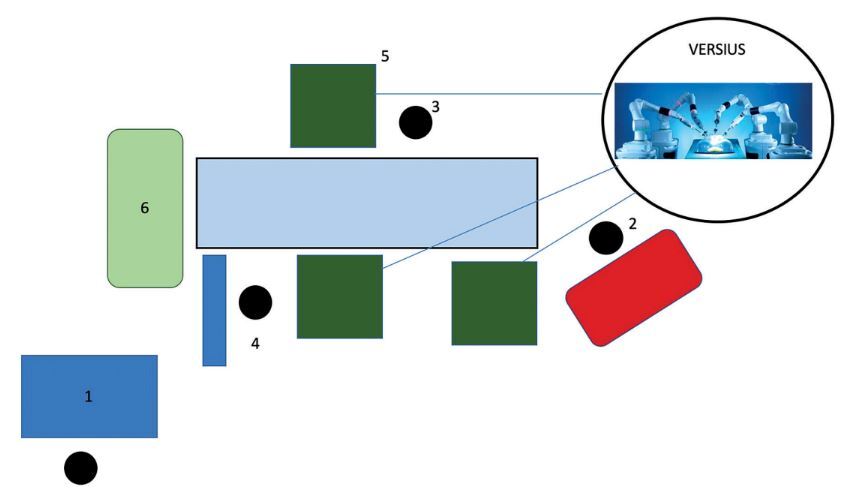
The surgeon sits at the console, which is usually positioned in close proximity to the patient (Figure 1). The robot’s vision system provides a real-time three- dimensional image of the surgical field, unlike conventional laparoscopy, which uses a two- dimensional image. The console allows the surgeon to simultaneously control two of the four surgical manipulators in which the instruments are mounted. The robotic arms replicate the surgeon’s movements, which are usually scaled down to maximize precision. The surgeon can switch between the arms and adjust the camera position at any time. Throughout the procedure, the main surgeon is assisted by a patient-side assistant who sits or stands directly next to the robot’s arms and is responsible for changing surgical instruments and making any necessary adjustments to the position of the robotic arms [4].
FIGURE 1
Operating room layout using the da Vinci robot. 1 – operator console, 2 – anesthesia machine, 3 – assistant surgeon, 4 – instru- mentation table, 5 – surgical manipulators, 6 – tower visualizing the surgical field
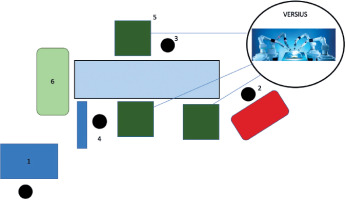
An alternative to the da Vinci system is provided by Versius robots manufactured by CMR Surgical, which have a very similar operating principle. The main difference between the two devices is the set of manipulators: in the Versius robot, each robotic arm is mounted on a separate, portable platform, which means they can be placed in any position relative to one another and to the patient (Figure 2). Additionally, the Versius robot allows up to seven arms to be used during one procedure, which are controlled simultaneously by two operators seated at two separate consoles [5].
FIGURE 2
Operating room layout using the Versius robot. 1 – operator console, 2 – anesthesia machine, 3 – assistant surgeon, 4 – instru- mentation table, 5 – surgical manipulators on separate platforms, 6 – tower visualizing the surgical field
It is worth emphasizing that both the da Vinci robot and the Versius robot are surgical manipulators controlled by a human. They are not capable of any autonomous or pre-programmed movements.
ANESTHESIA FOR ROBOT-ASSISTED SURGERY
Anesthetizing a patient for robotic surgery presents the anesthesiologist with a number of challenges related to the patient, the type of surgery, and the anesthesia itself.
Pneumoperitoneum and steep Trendelenburg position
In robotic surgery, as in laparoscopy, it is necessary to establish pneumoperitoneum with carbon dioxide. Gas insufflation elevates intra-abdominal pressure above physiological levels, which particularly affects the circulatory and respiratory systems during the procedure (Table 1) [4, 6]. In studies conducted on canine models, it has been observed that high intra-abdominal pressure (> 30 mmHg) decreases central venous pressure, increases systemic vascular resistance (SVR) and reduces cardiac output (CO) [7, 8]. It is worth emphasizing, however, that such high gas pressures are not used in medical applications. In clinical practice, the gas pressure usually does not exceed 12–15 mmHg [6, 9].
TABLE 1
Changes in parameters during steep Trendelenburg position and pneumoperitoneum
A study evaluating circulatory function during robotic prostatectomies showed that the creation of a pneumoperitoneum and Trendelenburg positioning (at 45o), as compared to measurements performed in the horizontal position, resulted in increased mean arterial pressure (MAP) (112.7 vs. 94.2 mmHg), elevated SVR (2014.1 vs. 1441.5 dyn s–1 cm–5), and slightly reduced cardiac output (4.9 vs. 5.3 L min–1), without significantly changing the heart rate and stroke volume [10].
The neuroendocrine response to the Trendelenburg position is characterized by an increase in nor-adrenaline levels and plasma NT-proANP concentrations due to atrial stretching caused by increased venous return [11].
During the procedure, changes in the respiratory system are also observed, caused by cephalad displacement of the diaphragm and abdominal contents. They include a fall in the functional residual capacity (FRC) of the lungs, decreased lung compliance, elevated airway resistance and peak airway pressure, which can lead to atelectasis and ventilation/perfusion mismatch [12]. The procedure may impair postoperative diaphragm function. Patients have also been observed to have elevated serum markers of lung damage after surgery [13].
Patient positioning and pneumoperitoneum may also affect intracranial pressure (ICP) and central nervous system perfusion (Table 1). A study using transcranial Doppler ultrasound in patients undergoing laparoscopic surgery in the Trendelenburg position showed that the mean ICP increased during the procedure [14]. It is worth noting, however, that cerebral perfusion pressure did not change statistically significantly [14]. Nevertheless, the literature describes cases of cerebral edema following cystectomy and robotic hysterectomy [15, 16]. The authors of these reports suggest that this complication is related to the long operation time, prolonged steep Trendelenburg position with high pressures of carbon dioxide insufflation, as well as liberal fluid therapy [15, 16].
The steep Trendelenburg position may also lead to increased intraocular pressure (IOP). This effect is further enhanced by an increase in the partial pressure of carbon dioxide (PaCO2) used as the insufflation gas to establish pneumoperitoneum [17]. Increased IOP is accompanied by decreased ocular perfusion pressure. This may lead to severe irreversible vision loss due to optic neuropathy or cause transient visual field defects [18, 19]. Other factors that may affect IOP include peak inspiratory pressure (PIP), MAP, and operation time [17, 20, 21]. The risk of increased IOP can be reduced by tilting the operating table at a smaller angle or using the modified Z Trendelenburg position, in which the patient’s head and shoulders are elevated to a horizontal (“zero”) position [22, 23]. The choice of anesthetic agents for general anesthesia also influences the level of IOP. The use of total intravenous anesthesia (TIVA) produces not only lower ICP but also lower IOP [24]. Other ophthalmological complications of robotic surgery include corneal damage and retinal detachment or tear [25].
Maintaining the patient in the steep Trendelenburg position with pneumoperitoneum for a long time also leads to increased swelling of the head (face) and neck area. If swelling occurs in the upper airways, patients may require prolonged intubation or re-intubation in the immediate postoperative period [26].
Air embolism
Robotic surgery, similarly to laparoscopic surgery, involves an increased risk of air embolism [27]. A carbon dioxide embolism probably occurs due to the passage of carbon dioxide into the damaged vessel during the rapid creation of pneumoperitoneum at high gas flow rates.
The anesthesiologist should be vigilant of the characteristic symptoms of gas embolism: a sudden rise and rapid fall in end-tidal carbon dioxide, sudden hypotension, tachycardia, cyanosis, decreased breath sounds, and a characteristic “mill wheel” murmur heard over the heart. The severity of symptoms depends on the size of the gas bubbles and the speed of their penetration into the bloodstream [6, 27, 28].
If symptoms of gas embolism occur, the anesthesiologist should immediately stop administering nitrous oxide if it is being used for gene ral anesthesia, start hyperventilation with 100% oxygen, rapidly remove the pneumoperitoneum, and place the patient in the left lateral decubitus and Trendelenburg positions. An attempt should be made to aspirate the gas bubbles through a central venous catheter or to insert a needle, aimed toward the left shoulder, directly into the right ventricle via a substernal approach [27].
The robotic procedures discussed here also pose the risk of paradoxical arterial embolism involving the brain and coronary vessels. This type of embolism is most common in patients with intracardiac septal defects or patent foramen ovale, which occurs in approximately 20–30% of the general population [6]. There are also reports of postoperative paralysis in patients undergoing robotic cystectomy, probably due to intraoperative cerebral air embolism [29].
Risk of hemorrhage
Robot-assisted procedures involve less blood loss during the surgery than conventional laparoscopic procedures (on average 164.2 mL for robot-assisted radical prostatectomy vs. 291.5 mL for laparoscopic radical prostatectomy) [30].
However, it should be noted that if hemorrhage occurs during a robotic procedure, it may be more difficult to control.
Risk of peripheral nerve damage
In Poland, most robot-assisted operations are urology procedures. They require special patient positioning – most commonly the steep (up to 30–45o)
Trendelenburg position. Such an extreme position combined with a long operation time may cause damage to the peripheral nerves, manifested by muscle weakness, pain, and paresthesia [26]. In a study of positioning injuries during robot-assisted urological procedures, the above symptoms of peripheral nerve damage were observed in 6.6% of patients [31].
Peripheral nerve injury may affect the upper limb (the brachial plexus), the lower limb (the femoral, obturator, and sciatic nerves, the lateral cutaneous nerve of the thigh, or the common peroneal nerve), as well as the lingual and buccal nerves [25]. One of the most frequently reported complications of robot-assisted surgery is brachial plexus injury caused by the use of “restraints” that prevent the patient placed in the steep Trendelenburg position from sliding down [26, 32]. Among the factors that may affect the occurrence of intraoperative peripheral nerve injuries, one can distinguish those that are directly related to the patient – such as their body build, body mass index (BMI), or a higher ASA (American Society of Anaesthesiology) score – and those related to the procedure itself – such as the required positioning of the patient, duration of the procedure, and experience of the surgical team [31].
Many authors believe that during positioning of the patient, care should be taken to protect the patient’s head (face), upper limbs and other parts of the body that may accidentally come into contact with the robotic arms. Most often, positioning kits consist of sponge-foam pads and cushions, usually used when positioning the patient for surgery in the prone position [33].
Hypothermia
Robot-assisted surgery typically takes longer than the same procedures performed laparoscopically. Operating time in robotic procedures depends on factors such as the experience of the surgical team, the learning curve, and robot docking time. Due to the longer duration of robotic procedures, patients run a greater risk of developing hypothermia and so should be protected against it using heating devices [6, 27, 32].
Limited patient access
One of the main problems an anesthesiolo-gist is confronted with during robot-assisted surgery is the lack of access to the patient as well as limited floor space for the anesthesia workstation (Table 2). The surgical robot takes up a large area in the operating room, most often directly next to the operating table. Another problem is that once the robotic arms are deployed and the tools are inserted into the patient’s body, they cannot be easily repositioned or removed, which is why it is important to ensure patient safety before the robot starts operating. The endotracheal tube, intravenous accesses, drains, the arterial line and patient monitoring devices – ECG, SpO2, blood pressure measurement, neuromuscular blockade monitoring (ToF), and depth of anesthesia monitoring – must all be secured in such a way as to minimize the risk of disconnection or kinking throughout the procedure. In addition, it is necessary to ensure easy access to the drug administration site [6].
In urgent or emergency situations, the robot can be rapidly removed from the patient’s body. Undocking is a multi-step procedure that requires deactivation of the robotic arms, sliding the tools out of the trocars and disconnecting them from the arms (in an order that depends on the type of device used). Only then can the patient or operating table be re-positioned without the risk of harming the patient [33]. Any medical center performing robot-assisted surgery should have a protocol in place for rapid undocking in emergency situations [1, 32].
Patient immobilization
The patient must remain motionless throughout the operation. Unlike conventional laparoscopy, robot-assisted surgery does not allow the surgeon to simultaneously control all the instruments in the patient’s body. The operator controls only two robotic arms at any given time. The others are held in a fixed position, which is why any uncontrolled movement on the patient’s part may lead to potentially life-threatening damage to anatomical structures. To prevent injury, deep neuromuscular blockade must be provided and monitored [6].
Depending on the type of surgery, the patient must be placed in a specific position to facilitate access to the surgical site. In many procedures, the steep Trendelenburg position is employed. To eliminate the risk of the patient sliding off the operating table or changing position, various types of safety supports, anti-skid egg crate foam pads or bean-bags are often used [6, 34, 35].
ROBOTIC SURGERY SPECIALTIES
Cardiac surgery
The first robot-assisted coronary artery bypass grafting surgery was performed in 1998 [36]. Robot-assisted procedures are associated with a shorter hospital stay, rapid return to daily activities, reduced pain since no sternotomy incision is required, and a potentially lower risk of massive transfusions of blood products [37]. The list of cardiac procedures performed using a robot is constantly growing. At the moment, the following procedures are performed with the assistance of a robotic system: aortic and mitral valve repair or replacement, atrial septal defect closure, total endovascular coronary artery bypass, minimally invasive direct coronary artery bypass, atrial fibrillation ablation, intracardiac tumor resection [38].
Robot-assisted cardiac surgery requires the insertion of instrument ports through the chest wall, collapse of one lung, insufflation of carbon dioxide into the chest, and isolated one-lung ventilation (OLV) [37]. The use of a double-lumen endotracheal tube or bronchial blocker and OLV during the procedure is necessary to provide space within the chest for the movement and operation of the robotic instruments. This means that patients are exposed to the hemodynamic and respiratory effects of OLV [38]. Carbon dioxide pneumothorax in the range of 5 to 10 mmHg allows visualization of cardiac structures but increases intrathoracic pressure and reduces venous return [39]. An important role in robotic procedures is played by the patient’s body habitus, especially the structure of the chest, since robot tools cannot be properly positioned if the thoracic surface area is too small.
That is why special care should be exercised in clearing patients for a robot-assisted procedure. Contraindications to OLV must be taken into account, which include severe pulmonary hypertension, suspected multiple pleural adhesions, recent myocardial infarction or unstable coronary artery disease, cor pulmonale, asthma, chronic obstructive pulmonary disease, and tuberculosis [38].
Thoracic surgery
Robot-assisted thoracic surgery is most often performed for the resection of lung, mediastinal or esophageal tumors [40]. The use of a surgical robot facilitates navigation and surgery in hard-to-reach spaces such as the mediastinum. As in other surgical specialties, the key to the success of robot-assisted thoracic surgery is proper patient clearance (Table 3). Appropriate positioning of the robot arms may be difficult or impossible in patients with a BMI above 35 kg m–2, as well as in very slim and short indivi duals [36]. Patients with a severe lung or heart disease are unlikely to tolerate OLV or the resulting changes in venous return. Moreover, patients who have undergone radiation therapy for cancer or thoracic surgery or those with a history of chest trauma may have multiple adhesions, which may pose technical difficulties for the operators [34]. Thoracic surgery procedures are most often performed in the lateral decubitus position, or in the supine position with the head tilted if mediastinum exposure is necessary [34].\
As with other robot-assisted procedures, before docking the robot, the anesthesiology team must check patient positioning and the securing of intravenous accesses, monitoring elements, and, above all, the endotracheal tube and the ventilator breathing system. It is particularly important to confirm the proper positioning of the double-lumen tube using a bronchofibroscope so that lung isolation is reliable and accurate. Bronchial blockers are less commonly utilized in such procedures because they might migrate into the trachea when the patient’s position is changed [34]. During OLV, the PaCO2 level increases due to the diffusion of insufflated carbon dioxide. Allowing hypercapnia to a pH > 7.25, avoiding high respiratory rates lest air trapping should occur, and using PEEP have been suggested as mitigating measures [40].
Carbon dioxide insufflation during robotic thoracic surgery may also cause compression of mediastinal structures such as the right atrium and vena cava, leading to reduced venous return and cardiac output [40]. These changes may be more severe when the right chest is insufflated. Transient increases in central venous pressure, pulmonary artery pressure, and pulmonary artery wedge pressure may also occur.
Rapid insufflation of carbon dioxide into the chest may give rise to symptoms of tension capnothorax. Stretching of the pleura can increase vagal tone, causing severe bradycardia, while compression of mediastinal vessels may lead to hypotension requiring treatment with IV fluids and vasoconstrictors. Decompression of the pleural cavity should be the first step in resolving such a situation [34].
Urology
Robot-assisted radical prostatectomies have been performed for over 20 years now. The first such operation was described in 2000 [41].
For the anesthesiologist, the main challenge of robotic urological procedures is the steep Trendelenburg position combined with the need to create pneumoperitoneum. The Trendelenburg position increases venous return, causing an increase in CO. On the other hand, insufflation of carbon dioxide and the establishment of pneumoperitoneum lead to compression of the aorta and increased SVR, which may result in decreased, rather than increased, SV and CO [10]. The combination of pneumoperitoneum and steep Trendelenburg positioning promotes a cephalad shift of abdominal contents, which leads to a reduction in lung compliance and FRC.
Pressure-controlled ventilation (PCV) has been recommended as a preferred ventilation method, as it allows for the use of lower pressures in the airways in order to maintain adequate minute ventilation and prevent the accumulation of carbon dioxide [42]. In patients placed in the Trendelenburg position, the placement of the endotracheal tube should be monitored as the trachea may be displaced in the cephalad direction, causing the endotracheal tube to fall deeper into a mainstem bronchus [43].
Gynecology
Gynecological procedures performed with the assistance of a surgical robot include sacrocolpopexy, hysterectomy, tubal reanastomosis, lymph node dissection, and myomectomy [44]. The use of robots in gynecological surgery is gaining popularity as it allows surgeons to perform procedures with high accuracy in the difficult-to-access and limited space of the pelvis [45]. Similarly to urological procedures, gynecological procedures require Trendelenburg positioning and the establishment of a pneumoperitoneum; hence, the problems faced by anesthesiologists are similar in both specialties. However, gynecological procedures usually require a less steep Trendelenburg position [1].
Head and neck surgery
The most commonly performed robot-assisted head and neck operations include tongue base re-section, radical tonsillectomy, partial pharyngectomy, laryngectomy, thyroid surgery, and neck lymph node removal [46, 47].
The assistance of a surgical robot in head and neck surgery significantly improves visualization of the surgical field and its accessibility. This, in many cases, eliminates the need for a tracheostomy. Moreover, patients undergoing robotic surgery often experience faster healing and a faster return to preoperative levels of functioning, including eating and speaking [46].
During head and neck operations, the patient is positioned with head 180o away from the anesthetic machine. The patient usually lies with the head tilted back and the shoulders raised. Immediately next to the operating table is the column with robot arms, which limits access of the anesthesiology team to the patient [46].
Intubation is most often performed using reinforced tubes or tubes adapted for laser procedures. Patients who have not undergone tracheostomy during surgery usually remain intubated in the postoperative period due to the significant risk of airway edema [46].
ANESTHETIC APPROACH FOR ROBOT-ASSISTED SURGERY
The choice of an anesthetic approach is always made by the anesthesiologist and is governed by their knowledge of the patient’s clinical condition, the type and duration of the procedure, and any potential complications.
There are no clear recommendations in the literature regarding the preferred anesthetic approach. Some authors suggest that general anesthesia with a volatile anesthetic should be used, even for thoracic surgery [4, 26, 32, 40]. However, TIVA may have an advantage over combined general anesthesia as it causes a smaller increase in IOP, which may be vital when the patient is maintained in the steep Trendelenburg position for an extended time [24].
In general, various authors recommend avoiding the use of nitrous oxide in robotic procedures. This gas may produce “intestinal distention” and may also exacerbate postoperative nausea and vomiting [32, 48].
Deep neuromuscular blockade or even continuous infusion of a muscle relaxant is recommended because patient coughing and movement can have serious consequences when the robot is operating [32]. The device does not adapt to the patient’s movements, which means that sudden tissue tension may result in tissue injury and serious bleeding [34].
The surgical approach that uses carbon dioxide insufflation to obtain space for the operation requires the anesthesiologist to carefully monitor the patient’s ventilation. Reduced lung compliance and FRC, higher airway resistance, and diffusion of insufflation gas into the blood result in a constant increase in end-tidal carbon dioxide levels and promote respiratory acidosis. PCV provides similar minute ventilation rates but is associated with lower PIP levels, thereby reducing the risk of lung injury [42]. It offers better lung compliance and better preserved ventilation-perfusion matching for the same levels of minute ventilation [49]. The use of lung protective ventilation, with tidal volumes of 6–8 mL kg–1, is recommended. It is also important to use adequate PEEP to limit atelectasis [13, 32].
The preferred monitoring method during robotic surgery is standard monitoring: ECG, SpO2, blood pressure, and assessment of depth of anesthesia and neuromuscular blockade. Blood pressure is often monitored using an invasive method, which provides real-time measurements and makes it easy to determine arterial blood gases during surgery [26, 34, 40]. Central vascular catheters are not routinely used and are reserved for patients with severe comorbidities. Instead, peripheral vascular access devices are utilized: at least two, on both upper limbs, that can be easily accessed during the procedure [34, 40].
Due to the significant changes in the circulatory system occurring during robotic surgery, the use of hemodynamic monitoring is also worth considering. In studies assessing these changes, the authors used both advanced methods enabling transpulmo-nary arterial thermodilution and invasive monitoring of blood pressure with minimally invasive monitoring of cardiac output [50–54].
SAFETY OF ROBOT-ASSISTED SURGERY
Robotic procedures are minimally invasive in terms of procedural technique. However, because during those procedures the patient is maintained in a specific position often for prolonged periods of time, they significantly interfere with human physiology. For this reason, patients should be carefully selected for robotic surgery to ensure that it does not pose too great a risk to their body’s homeostasis. Increased-risk patients include those with comorbid cardiovascular diseases, poor lung function, pulmonary hypertension, cerebrovascular diseases, and glaucoma [55].
Although the steep Trendelenburg position is known to cause numerous changes in the body’s physiology, its influence on the incidence of perioperative complications remains unclear. A 2022 meta-analysis of 10 randomized and 47 non-randomized studies of postoperative complications of the steep Trendelenburg position, involving a total of 380,125 patients, showed that robot-assisted surgery was not associated with an increased risk of thromboembolic events, circulatory complications, or central nervous system vascular events [58].