For the past 25 years [1], the obesity paradox has been documented in the medical literature. It describes the phenomenon of higher survival rates among obese and overweight patients in comparison to normal- and underweight counterparts, with underweight patients often showing the highest mortality rates. This paradox has been mainly investigated in sepsis for nearly 15 years and has been the focus of multiple systematic reviews and meta-analyses [2–8].
Despite extensive research in clinical settings, a definitive conclusion regarding the validity of the obesity paradox remains elusive [2–8]. Evaluating this phenomenon in preclinical models offers a controlled environment to study the complex interactions between obesity and sepsis outcomes, potentially reducing the heterogeneity of the study group. However, the results of individual studies can vary due to differences in design-related variables, which can significantly influence survival outcomes [9].
Furthermore, the physiological differences between humans and mice, particularly in immune system function and metabolism, add another layer of complexity to interpreting these findings [10, 11]. Nevertheless, given the significant heterogeneity and multimorbidity among sepsis patients [12, 13], the murine sepsis-obesity model offers a promising approach to investigate the validity of the obesity paradox in sepsis.
Sepsis in humans is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [14]. In animal studies, sepsis is typically modelled using three main approaches: injection of a toxic agent, injection of live pathogens, and impairment of barrier tissue integrity. The first two methods are generally minimally invasive and non-surgical, while the third often requires surgical intervention [15–17]. Overweight and obesity are classified as abnormal or excessive fat accumulation that poses a health risk, with a body mass index (BMI) over 25 kg m–2 considered overweight and over 30 kg m–2 classified as obese [18]. Animal models of obesity can be broadly divided into two categories: those based on genetic mutations or manipulations, and those based on exposure to obesity-promoting factors, such as a high-fat diet (HFD) [19, 20].
METHODS
Aim of the study
The aim of the study was to systematically review and meta-analyze published research on the obesity paradox in murine sepsis models, specifically focusing on the relationship between obesity and survival outcomes.
Literature search
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21]. A PRISMA flow diagram detailing the study selection process is presented in Figure 1. Articles were searched for in the PubMed/ Medline database. The search included articles available until January 31, 2025 (inclusive). The following search criteria were used: ((sepsis[Title/Abstract]) OR (septic[Title/Abstract])) AND ((obesity[Title/ Abstract]) OR (obese[Title/Abstract]) OR (fat[Title/ Abstract]) OR (adipose[Title/Abstract]) OR (body mass[Title/Abstract])) AND ((animal[Title/Abstract]) OR (mouse[Title/Abstract]) OR (mice[Title/Abstract]) OR (murine[Title/Abstract])).
The screening and selection of studies were performed by three researchers. Two team members independently screened titles and abstracts for eligibility, and full texts of potentially relevant studies were assessed against predefined inclusion criteria. A third investigator supervised the process, resolved discrepancies, and ensured methodological consistency. Studies were included if they met the following eligibility criteria: (1) murine model of sepsis, (2) septic mice without obesity as a control group, and (3) available mortality data for both groups. All studies meeting these criteria were included in the systematic review, regardless of the specific sepsis or obesity model used. Each distinct survival assessment – defined by a unique pairing of obesity-induction method, HFD variant, and sepsis model – was counted as an individual experiment, even when reported within the same publication, to capture within-study heterogeneity.
For the meta-analysis, only studies in which sepsis was induced using the cecal ligation and puncture (CLP) method and obesity was obtained using the diet-induced obesity (DIO) model were included. These models were the most frequently used, allowing for standardization of the analysis in terms of sepsis and obesity induction. To comprehensively assess the impact of HFD duration on survival in sepsis, studies involving short-term HFD administration were also included in both the systematic review and meta-analysis.
Data extraction
Data extraction was performed independently by two researchers using a predefined extraction form. A third investigator oversaw the process, verified the extracted data, and resolved any discrepancies. For each survival experiment, the primary outcome was mortality, assessed as the number of individuals reported dead by the end of the investigator-defined period.
Additionally, data related to the obesity model used, sepsis model, sex, age at the start of HFD administration (in the case of DIO), duration of HFD administration, age at sepsis induction, observation time, and diet composition were extracted. When necessary, corresponding authors of the included studies were contacted to clarify or obtain missing data.
Statistical analysis
Meta-analysis and meta-regression were performed using Statistica software (v13.3, StatSoft, Tulsa, USA). Results were considered statistically significant at P < 0.05. For each study included in the meta-analysis, the odds ratio (OR) based on mortality data was calculated to assess the effect of obesity and other covariates. A random-effects model was used, and heterogeneity was evaluated using Cochran’s Q test (P < 0.05) and the I2 statistic. Potential publication bias was examined via funnel plots, Egger’s test (P < 0.05), and the trim-and-fill method. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework was applied to assess the overall certainty of evidence, considering risk of bias, inconsistency, indirectness, imprecision, and publication bias [22].
RESULTS
A total of 392 records were identified via a systematic search of the database for articles published before January 31, 2025, and screened for inclusion based on eligibility criteria. Titles and abstracts of all articles were reviewed, and 41 potentially eligible articles were retrieved. The full text was then reviewed, and 21 studies were selected that met the inclusion criteria for the systematic review. Ten of them used the CLP method to induce sepsis and HFD for the induction of obesity (CLP-DIO sepsisobesity model) and were included in the meta-analysis. The study selection process is presented in Figure 1.
SYSTEMATIC REVIEW
Study characteristics and quality assessment
The systematic review included 21 studies that assessed survival in the murine model of sepsis in a total of 38 experiments [23–43]. CLP was used to induce sepsis in 14 experiments (13 studies). In the remaining experiments, sepsis was induced by intraperitoneal administration of lipopolysaccha-ride (LPS) (7 experiments/5 studies), intravenous administration of Staphylococcus aureus LS-1 (8/2), intraperitoneal administration of Pseudomonas aeruginosa (4/1), intraperitoneal administration of Salmonella typhimurium (4/1), and intraperitoneal administration of cecal slurry (CS) (1/1).
In 33 experiments (18 studies), HFD was used; in 3 experiments (3 studies), obesity was achieved by subcutaneous injection of monosodium glutamate (MSG) in the neonatal period; and in 2 experiments (2 studies), obesity was induced through genetic modification (leptin-deficient). Two studies used more than one obesity model. The HFD used in the studies differed in composition, fat content, and percentage of energy from fat depending on the study (39.1–61.1%). The diets used in the control group also differed regarding the above parameters (percentage of energy from fat 9.0–18.0%). Some studies assessed the impact of HFDs with various compositions on survival, primarily focusing on the type of fat used. Moreover, in individual studies, the animals differed in age at the initiation of the HFD (42–112 weeks), duration of its use (3–172 days), and age of sepsis induction (58–224 weeks). Depending on the study, the observation period to assess survival ranged from 30 to 672 hours. Detailed information for the studies included in the systematic review is summarized in Table 1.
TABLE 1
Characteristics of studies included in the systematic review.
| Ref. | Experimental group (obese) | Control group (lean) | Sepsis model | Start feeding age (days) | Feeding duration (days) | Sepsis induction age (days) | Observation duration (hours) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Sex | Obesity model | kcal % FAT | n | Mortality % | Strain | Sex | kcal % FAT | n | Mortality % | ||||||
| Berto-Pereira et al., 2024 [23] | Swiss mice | F | MSG | n/d | 16 | 81.3 | Swiss mice | F | n/d | 21 | 100.0 | CLP | n/a | n/a | 75 | 168 |
| Nakama et al., 2024 [24]a | Swiss mice | M | MSG | 11.5 | 22 | ~40.0 | Swiss mice | M | 11.5 | 48 | ~80.0 | CLP | n/a | n/a | 75 | 168 |
| Nishimura et al., 2023 [25]a | KCASP1Tg | F | DIO | 60.0 | 5 | 60.0 | KCASP1Tg | F | n/d | 5 | 100.0 | LPS (500 μg i.p.) | 112 | 56 | 168 | 36 |
| C57BL/6N | n/d | DIO | 60.0 | 5 | 0.0 | C57BL/6N | n/d | n/d | 5 | 0.0 | LPS (500 μg i.p.) | n/d | 56 | n/d | 36 | |
| Bodilly et al., 2023 [26] | C57BL/6 | M | DIO | 61.1 | 16 | 100.0 | C57BL/6 | M | 16.5 | 16 | 100.0 | CLP | 42 | 49 | 91 | 96 |
| Gomes et al., 2023 [27] | C57BL/6 | F | DIO | 39.2 | 24 | 41.7 | C57BL/6 | F | 9.5 | 21 | 13.1 | CLP | 21-28 | 98 | 119–126 | 168 |
| Petroni et al., 2022 [28] | C57BL/6 | M | DIO | 60.0 | 15 | 93.3 | C57BL/6 | M | 11.5 | 15 | 86.7 | LPS (10 mg/kg i.p.) | 56 | 42 | 98 | 72 |
| Vankrunkelsven et al., 2022 [29] | B6.V-Lepob/ob/JRj | M | leptin KO | 10.0 | 30 | 53.3 | C57BL/6J | M | 9.0 | 18 | 38.9 | CLP | 42 | 77–84 | 119–126 | 125 |
| C57BL/6J | M | DIO | 60.0 | 26 | 23.1 | |||||||||||
| Lewis et al., 2022 [30] | C57BL/6 | M | DIO | 60.0 | 18 | 33.3 | C57BL/6 | M | 10.0 | 20 | 75.0 | CS (500 μl CS i.p.) | 42 | 140–147 | 182–189 | 336 |
| Martins et al., 2021 [31] | Swiss mice | M | DIO | 60.2 | 6 | 100.0 | Swiss mice | M | 11.5 | 5 | 0.0 | CLP | 56 | 3 | 59 | 96 |
| Nakama et al., 2021 [32] | Swiss mice | M | MSG | 11.5 | 20 | 5.0 | Swiss mice | M | 11.5 | 20 | 45.0 | CLP | n/a | n/a | 75 | 168 |
| Wang et al., 2021 [33]a | C57BL/6 | M | DIO | 60.0 | 11 | 100.0 | C57BL/6 | M | 10.0 | 11 | 90.9 | LPS (10 mg/kg i.p.) | 28 | 105 | 133 | 72 |
| Souza et al., 2019 [34]b | Swiss mice | M | DIO | 60.0 | 5 | 100.0 | Swiss mice | M | 10.0 | 7 | 100.0 | LPS (30 mg/kg i.p.) | 56 | 3 | 59 | 54 |
| Swiss mice | M | DIO | 60.0 | 6 | 100.0 | Swiss mice | M | 10.0 | 6 | 50.0 | CLP | 56 | 3 | 59 | 150 | |
| Napier et al., 2019 [35]a | BALB/c | F | DIO | 42.0 | n/d | 100.0 | BALB/c | F | 18.0 | n/d | ~17.0 | LPS (6 mg/kg i.p.) | 42-56 | 16 | 58–72 | 100 |
| BALB/c | F | DIO | 42.0 | n/d | 100.0 | BALB/c | F | 18.0 | n/d | 100.0 | LPS (8 mg/kg i.p.) | 42-56 | 16 | 58–72 | 80 | |
| Frydrych et al., 2019 [36] | C57BL/6 C57BL/6J | M | DIO | 60.0 | 20 | 60.0 | C57BL/6 | M | 13.0 | 20 | 20.0 | CLP | 42 | 154–172 | 196–224 | 672 |
| Kaplan et al., 2016 [37]a | C57BL/6 | M | DIO | 61.1 | 12 | 83.3 | C57BL/6 | M | 16.5 | 12 | 66.7 | CLP | 42 | 42–49 | 84–91 | 30 |
| Siegl et al., 2015 [38] | C57BL/6J | M | DIO | 54.0 | 24 | 0.0 | C57BL/6J | M | 11.0 | 30 | 20.0 | CLP | 49 | 84 | 133 | 48 |
| Svahn et al., 2015 [39] | C57BL/6 | M | DIO | 60.0 S P : C -1 : 1 | 20 | 80.0 | C57BL/6 | M | 10.0 | 20 | 35.0 | Staphylococcus aureus LS-1 (0.2 ml i.v.) | 49 | 56 | 105 | 408 |
| C57BL/6 | M | DIO | 60.0 P P : C-1 : 1 | 20 | 15.0 | |||||||||||
| C57BL/6 | M | DIO | 60.0 S P : C -3 : 1 | 20 | 60.0 | |||||||||||
| C57BL/6 | M | DIO | 60.0 P P : C-3 : 1 | 20 | 20.0 | |||||||||||
| C57BL/6 | M | DIO | 60.0 S P : C-1 : 3 | 20 | 60.0 | |||||||||||
| C57BL/6 | M | DIO | 60.0 P P : C-1 : 3 | 20 | 15.0 | |||||||||||
| Siegl et al., 2014 [40] | C57BL/6J | M | DIO | 54.0 | 14 | 28.6 | C57BL/6J | M | 11.0 | 10 | 90.0 | CLP | 49 | 84 | 133 | 240 |
| Kaplan et al., 2012 [41]a | C57BL/6 | M | DIO | 61.1 | 12 | 91.7 | C57BL/6 | M | 16.5 | 12 | 50.0 | CLP | 42 | 21 | 63 | 30 |
| Strandberg et al., 2009 [42]a | C57BL/6 | M | DIO | 58.0 and 60.0 | 18 | 55.6 | C57BL/6 | M | 10.8 and 10.0 | 21 | 14.3 | Staphylococcus aureus LS-1 (0.2 ml i.v.) | 42–56 | 56 | 98–112 | 408 |
| Ob/Ob | M | leptin KO | 10.8 and 10.0 | 18 | 72.2 | C57BL/6 | M | 10.8 and 10.0 | 15 | 20.0 | Staphylococcus aureus LS-1 (0.2 ml i.v.) | 42–56 | 56 | 98–112 | 408 | |
| Clouva-Molyvdas et al., 1992 [43] | CF1 | F | DIO | 39.1 coconut oil | 30 | 26.7 | CF1 | F | 11.7 | 20 | 30.0 | Pseudomonas aeruginosa (i.p.) | n/d | 14 | n/d | 168 |
| CF1 | F | DIO | 39.1 safflower oil | 30 | 20.0 | |||||||||||
| CF1 | F | DIO | 39.1 oleic acid | 30 | 26.7 | |||||||||||
| CF1 | F | DIO | 39.1 MaxEPA oil | 30 | 20.0 | |||||||||||
| CF1 | F | DIO | 39.1 coconut oil | 31 | 71.0 | CF1 | F | 11.7 | 31 | 58.1 | Salmonella typhimurium (i.p.) | n/d | 21 | n/d | 336 | |
| CF1 | F | DIO | 39.1 safflower oil | 31 | 61.3 | |||||||||||
| CF1 | F | DIO | 39.1 oleic acid | 30 | 66.7 | |||||||||||
| CF1 | F | DIO | 39.1 MaxEPA oil | 31 | 54.8 | |||||||||||
M – male, F – female, DIO – diet-induced obesity, S – saturated, P – polyunsaturated, P : C – ratio of calories from protein to calories from carbohydrates, KO – knockout, MSG – monosodium glutamate, CLP – cecal ligation and puncture, LPS – lipopolysaccharide, CS – cecal slurry, i.v. – intravenous, i.p. – intraperitoneal, n – sample size, n/a – not applicable, n/d – no data.
Impact of obesity on survival
In the evaluated studies, mortality in the group with obesity (or short-term HFD use) was higher than in the control group in 18 experiments, equal in 4 experiments, and lower in 16 experiments.
Two studies examined the effect of HFD composition on survival in sepsis. Svahn et al. [39] assessed the impact of a research diet administered for eight weeks and found that the difference in fat composition influenced survival. Mice fed a polyunsaturated HFD had increased survival during sepsis compared with mice fed a saturated HFD, while differences in the proportion of dietary protein and carbohydrates did not affect septic survival. Clouva-Molyvdas et al. [43] used two models of sepsis (one with Pseudomonas aeruginosa and the other with Salmonella typhimurium), in which the animals were fed an HFD for 2 and 3 weeks, respectively, before infection. In both models, four different HFDs differing in the source of fat and a control diet were used. No significant differences were observed in survival among groups fed various levels and fat sources.
Two studies assessed sepsis survival in the DIO model and the genetic (leptin-deficient) model. In the study by Vankrunkelsven et al. [29], after five days of sepsis, mortality was highest in leptin- deficient mice (P = 0.03 vs. DIO) but not significantly different between control and DIO mice. Moreover, in this study, despite similar body masses (DIO mice 43.9 ± 4.7 g, leptin-deficient mice 44.4 ± 2.8 g, P = 0.5), leptin-deficient mice had higher fat mass but lower lean body mass than obese DIO mice (P < 0.0001 for both). Strandberg et al. [42] reported that C57BL/6 mice on an HFD for eight weeks, like genetically obese mice on a low-fat diet (LFD), had increased mortality during Staphylococcus aureus-induced sepsis compared with LFD-fed C57BL/6 controls. They also found that the mortality in C57BL/6 mice fed an HFD throughout the entire experiment was higher than in mice on an LFD throughout the whole period (P = 0.02). There was no increase in mortality when comparing mice that had been switched from LFD to HFD on the day of staphylococcal inoculation with mice that had been fed an LFD throughout. Moreover, there was no significant difference in mortality between mice fed an HFD before inoculation and switched to an LFD on the day of inoculation and mice on an HFD throughout [42].
Rationale for performing a meta-analysis and for model selection
Differences in mortality between obese and control groups across various studies suggest that additional factors may influence outcomes. As noted earlier, the range of mouse age at the start of HFD feeding, the duration of feeding, the age at sepsis induction, and the observation period varied considerably among the reviewed studies. Moreover, the systematic review revealed that CLP was the most frequently used sepsis model, whereas HFD was the most common approach to inducing obesity. Hence, a meta-analysis was carried out exclusively on studies employing this combination of the most frequently used models (CLP-DIO) to reduce variability and reveal additional factors affecting mortality. By selecting the most widely utilized sepsis and obesity induction methods, the intention was to minimize discrepancies arising from differing obesity mechanisms and sepsis induction protocols.
Meta-analysis and meta-regression
Meta-analysis
To obtain a homogeneous group for the meta-analysis, studies were selected in which sepsis was induced by CLP and obesity by the DIO model. Ten studies met these criteria, involving 159 DIO mice and 149 control mice. They are summarized in Table 2. Eight studies used C57BL/6 mice, including four C57BL/6J mice. The remaining two studies used Swiss mice. In nine studies, the mice were male. Six experiments used commercial high-fat formulas with publicly available fatty-acid profiles (58Y1 n = 3, E15186-34 n = 2, E15742-34 n = 1); three employed modified high-fat versions of AIN-93 (AIN-93M n = 2, AIN-93G n = 1), and one used an unnamed HFD without further compositional details, precluding analysis of fat-type effects. Figure 2 presents, for each study, the age at HFD initiation, the duration of HFD feeding, and the age at sepsis induction.
FIGURE 2
Comparison of the duration of individual periods (pre-research diet feeding period, research diet feeding period, and observation period after sepsis induction) in the studies included in the meta-analysis
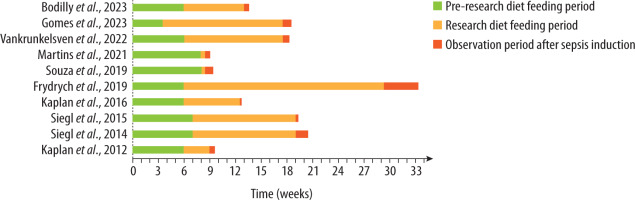
TABLE 2
Characteristics of studies included in the meta-analysis
| Ref. | Experimental group (obese) | Control group (lean) | Start feeding age (days) | Feeding duration (days) | Sepsis induction age (days) | Observa-tion duration (hours) | Needle gauge [G] | Pun-ctures (n) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Sex | Energy [kcal/g] | Kcal % FAT | Kcal % PRO | Kcal % CHO | Total (n) | Mortality % | Strain | Sex | Energy [kcal/g] | Kcal % FAT | Kcal % PRO | Kcal % CHO | Total (n) | Mortality % | |||||||
| Bodilly et al., 2023 [26] | C57BL/6 | M | 5.10 | 61.1 | 18.1 | 20.3 | 16 | 100.0 | C57BL/6 | M | 3.54 | 16.5 | 26.6 | 56.8 | 16 | 100.0 | 42 | 49 | 91 | 96 | 21 | 2 |
| Gomes et al., 2023 [27] | C57BL/6 | F | 4.59 | 39.2 | 13.5 | 47.3 | 24 | 41.7 | C57BL/6 | F | 3.79 | 9.5 | 16.3 | 74.2 | 21 | 13.1 | 21–28 | 98 | 119–126 | 168 | 21 | 1 |
| Vankrunkelsven et al., 2022 [29] | C57BL/6J | M | 5.15 | 60.0 | 20.0 | 20.0 | 26 | 23.1 | C57BL/6J | M | 3.23 | 9.0 | 24.0 | 67.0 | 18 | 38.9 | 42 | 77–84 | 119–126 | 125 | 18 | 2 |
| Martins et al., 2021 [31] | Swiss mice | M | 5.20 | 60.2 | 19.9 | 19.9 | 6 | 100.0 | Swiss mice | M | 3.50 | 11.5 | 25.7 | 62.8 | 5 | 0.0 | 56 | 3 | 59 | 96 | 18 | 1 |
| Souza et al., 2019 [34]a | Swiss mice | M | 5.20 | 60.2 | 19.9 | 19.9 | 6 | 100.0 | Swiss mice | M | 3.50 | 11.5 | 25.7 | 62.8 | 6 | 50.0 | 56 | 3 | 59 | 150 | n/d | n/d |
| Frydrych et al., 2019 [36] | C57BL/6 and C57BL/6J | M | n/d | 60.0 | n/d | n/d | 20 | 60.0 | C57BL/6 | M | n/d | 13.0 | n/d | n/d | 20 | 20.0 | 42 | 154–172 | 196–224 | 672 | 20 | 2 |
| Kaplan et al., 2016 [37]b | C57BL/6 | M | 5.10 | 61.1 | 18.1 | 20.3 | 12 | 83.3 | C57BL/6 | M | 3.54 | 16.5 | 26.6 | 56.8 | 12 | 66.7 | 42 | 42–49 | 84–91 | 30 | 22 | 2 |
| Siegl et al.,2015 [38] | C57BL/6J | M | 4.97 | 54.0 | 17.0 | 29.0 | 24 | 0.0 | C57BL/6J | M | n/d | 11.0 | 23.0 | 65.0 | 30 | 20.0 | 49 | 84 | 133 | 48 | 23 | 1 |
| Siegl et al., 2014 [40] | C57BL/6J | M | 4.97 | 54.0 | 17.0 | 29.0 | 14 | 28.6 | C57BL/6J | M | n/d | 11.0 | 23.0 | 65.0 | 10 | 90.0 | 49 | 84 | 133 | 240 | 23 | 1 |
| Kaplan et al., 2012 [41]b | C57BL/6 | M | 5.10 | 61.1 | 18.1 | 20.3 | 12 | 91.7 | C57BL/6 | M | 3.54 | 16.5 | 26.6 | 56.8 | 12 | 50.0 | 42 | 21 | 63 | 30 | 21 | 2 |
M – male, F – female, n/d – no data, Kcal% FAT – percentage of total calories derived from fat, Kcal% PRO – percentage of total calories derived from protein, Kcal% CHO – percentage of total calories derived from carbohydrates.
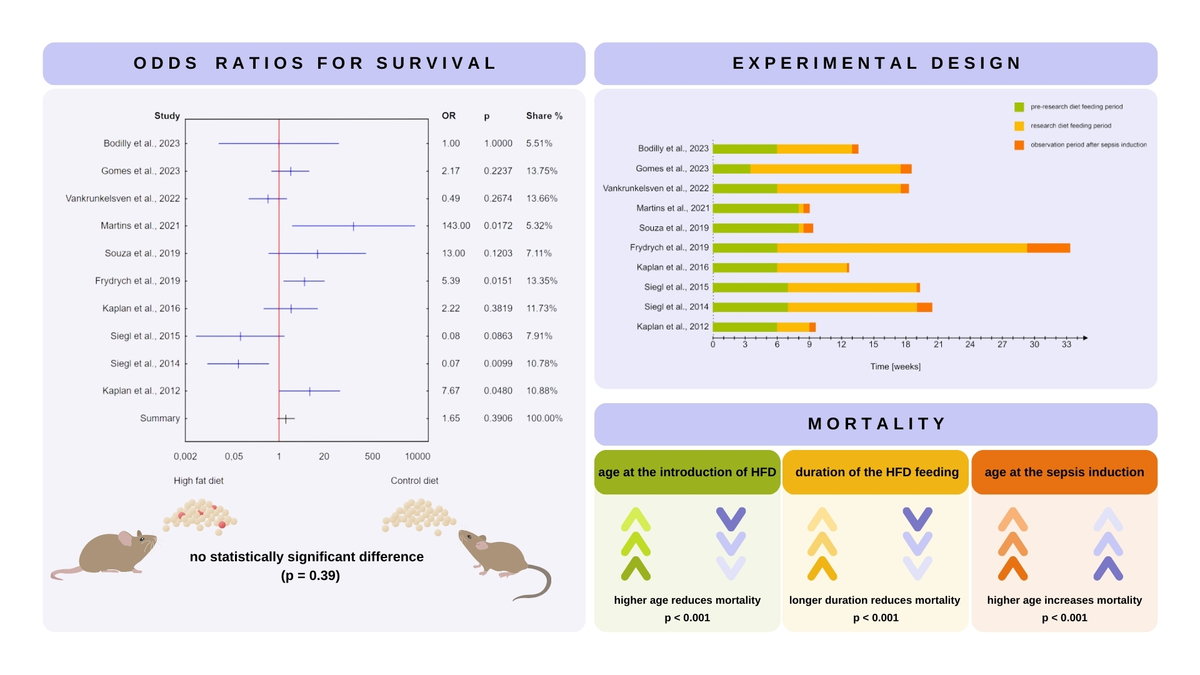
There was no statistically significant difference between animals receiving an HFD and those receiving a control diet (OR = 1.65, 95% CI: 0.53–5.19; P = 0.391), as illustrated in the forest plot presented in Figure 3.
FIGURE 3
Forest plot displaying the odds ratios (OR) and 95% confidence intervals for survival in mice fed a high-fat diet (HFD) compared to a control diet in the murine model of sepsis across the studies included in the meta-analysis. There was no statistically significant difference between the groups (P = 0.391)
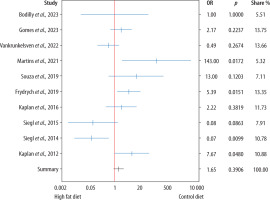
To assess the robustness of this result, a leave-one-out sensitivity analysis was performed. In this analysis, each study was sequentially excluded and the pooled effect size was recalculated. The resulting ORs ranged from 1.29 (when excluding Martins et al., 2021 [32]) to 2.35 (when excluding Siegl et al., 2014 [41]). In all cases, the direction and statistical significance of the pooled estimate remained unchanged (OR = 1.65, 95% CI: 0.53–5.19; P = 0.391), indicating that no individual study unduly influenced the overall result.
Meta-regression
The statistical meta-regression analysis showed that a higher age at the time of introduction of an HFD (P < 0.001) and a longer duration of feeding with an HFD (P < 0.001) reduce mortality. A higher age (P < 0.001) at sepsis induction increases mortality. In the above model, the regression coefficient R2 is 0.86 (P < 0.001). Figure 4 presents univariate analyses that illustrate the individual impact of these factors. In addition, the percentage of dietary energy from fat was evaluated as a potential predictor of survival; however, it was not significantly associated with mortality (P = 0.1016).
FIGURE 4
Univariate meta-regression analyses illustrate the individual impact of age at the initiation of a high-fat diet (HFD) (A), duration of HFD (B), and age at sepsis induction (C) on survival in murine sepsis models. These analyses demonstrate the independent influence of each factor. In the multivariate analysis presented in the Results section, these effects are moderated by the interactions between variables, leading to different outcomes when all factors are considered simultaneously

Heterogeneity and publication bias
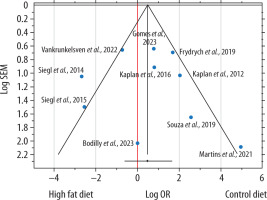
A moderate-to-high level of heterogeneity was observed (Cochran’s Q P = 0.0007, I2 = 68.6%), supporting the use of a random-effects model. A funnel plot was constructed to assess potential small-study effects (Figure 5). Although a few studies appear outside the main funnel boundary and may be considered outliers, overall visual inspection did not indicate substantial asymmetry, and Egger’s regression test (P = 0.7896) confirmed the absence of statistically significant publication bias. The trim-and-fill method (P = 0.3906) further indicated that no additional “missing” studies needed to be imputed, supporting the robustness of the meta-analytic findings.
FIGURE 5
Funnel plot of the studies included in the meta-analysis, assessing the association between a high-fat diet and mortality in murine sepsis models. The x-axis shows the log odds ratio (OR), with negative values favoring the high-fat diet group, and the y-axis shows the standard error of the mean (SEM). The vertical red line represents the pooled effect estimate, and the diagonal lines correspond to the pseudo 95% confidence intervals around this estimate. Although a few studies appear outside these boundaries and may be considered outliers, the overall distribution is relatively symmetrical, suggesting no definitive evidence of publication bias
GRADE assessment
The overall certainty of the evidence from the included studies was evaluated using the GRADE framework. As summarized in Table 3, the risk of bias was judged as moderate, heterogeneity as low, indirectness as low, imprecision as high (due to wide confidence intervals including 1), and publication bias as moderate. These ratings underscore both the variability in study designs and the relatively wide confidence intervals observed in the pooled analyses.
TABLE 3
GRADE assessment of the certainty of evidence
DISCUSSION
The obesity paradox remains a complex and intriguing phenomenon within medical research. Our systematic review and meta-analysis aimed to elucidate the relationship between obesity and survival outcomes in murine models of sepsis. The findings demonstrate significant variability in mortality outcomes, with some studies showing a survival benefit in obese mice while others do not.
The studies evaluated revealed mixed results, with obesity associated with higher mortality in 18 experiments, equal mortality in 4, and lower mortality in 16 experiments. This variability suggests that factors beyond obesity may be crucial in determining outcomes. The research by Svahn et al. [39] and Clouva-Molyvdas et al. [43] highlights the significant impact of dietary composition on survival outcomes. Mice fed polyunsaturated fats exhibited improved survival rates compared to those on saturated fats, underscoring the importance of nutritional components beyond caloric content. The comparison of DIO and genetic (leptin-deficient) models by Vankrunkelsven et al. [29] emphasizes the complexity of obesity as a phenotype. Despite similar body masses, leptin-deficient mice demonstrated differences in fat and lean mass composition, affecting survival outcomes. Obesity’s impact on the immune response, as shown in studies by Lewis et al. [30] and Strandberg et al. [42], further the relationship between obesity and sepsis survival. Altered cytokine profiles and immune cell functions in obese mice may contribute to differential survival outcomes [30, 42].
The results of the meta-analysis provide essential insights into the relationship between obesity and survival in murine models of sepsis, specifically those utilizing the CLP-DIO model. The lack of a statistically significant difference in mortality between the HFD and control groups (P = 0.391) suggests that obesity, as induced by an HFD, does not uniformly affect survival in sepsis. However, the statistical regression analysis identified critical variables influencing outcomes: higher age at the start of the HFD and longer duration of diet administration were associated with reduced mortality, while higher age at sepsis induction was linked to increased mortality. These findings underscore the importance of time-dependent factors in modulating the effects of obesity on sepsis outcomes and suggest that the obesity paradox may be context-dependent, varying with the timing of dietary intervention and the age at which sepsis is induced.
Despite observing a substantial degree of heterogeneity (I2 = 68.6%), our analyses did not reveal significant publication bias. The funnel plot showed no marked asymmetry, and Egger’s test (P = 0.7896), together with the trim-and-fill method (P = 0.3906), indicated that no additional “missing” studies were necessary to adjust the pooled estimate. These findings suggest that, despite variability in study protocols, the overall conclusions from our meta-analysis remain robust to small-study effects. Furthermore, the GRADE framework highlighted both the strengths and limitations of the current evidence. Although the risk of bias was rated as moderate and indirectness as low, imprecision was high due to wide confidence intervals that frequently crossed the line of no effect. This underscores that the pooled result from the meta-analysis may be less conclusive on its own. Indeed, meta-regression – pinpointing the importance of the age at HFD initiation, HFD duration, and the timing of sepsis induction – proved more revealing than the overall forest plot estimate. Such findings underscore the complexity of the obesity paradox in murine sepsis models and emphasize the need to consider time-dependent factors both when designing experiments and when interpreting the impact of obesity on survival in these experimental conditions.
The impact of obesity on outcomes in preclinical animal models of sepsis has already been the subject of comprehensive reviews, with detailed analyses provided by Xu et al. [44], and Eng et al. [9]. Xu et al. [44], in a systematic review and meta-analysis published in 2020, evaluated 21 studies comparing survival in obese versus non-obese animals (mice or rats) following exposure to bacteria, lipopolysaccharide, or influenza virus. The studies included in the comparison utilized various models of sepsis, infection, and obesity. Their analysis demonstrated that obesity consistently reduced survival in both single-strain bacteria- and lipopolysaccharide-exposed studies, not significantly in CLP models, and significantly in influenza models, albeit with high heterogeneity. Eng et al. [9], in a scoping review published in 2024, provided critical insights by analyzing the diversity of diet-induced sepsisobesity murine models, focusing on differences in sepsis induction (such as variable induction protocols, needle gauge, number of punctures), fluid resuscitation, antibiotic therapy, and analgesic administration, as well as variations in obesity models, particularly concerning the composition of HFDs [9]. These reviews have significantly advanced the understanding of the complexities of modelling sepsis and obesity in preclinical settings. To date, this paper is the first meta-analysis to assess the impact of time points and specific interventions, such as the age at the start of HFD, the duration of HFD, and the age at the time of sepsis induction, on survival in the CLP-DIO (sepsis-obesity) model.
The current body of research on the obesity paradox in murine models of sepsis is characterized by significant heterogeneity in experimental design. The most commonly used approach appears to be the CLP-DIO sepsis-obesity model; however, the composition of the HFD and the CLP procedures varies across studies. Our study and others addressing the sepsis-obesity murine model underscore the need for standardization. Additionally, our meta-analysis suggests that factors such as the age of the animals at the start of HFD, the duration of HFD, and the age at sepsis induction may significantly influence the observed impact of obesity on sepsis survival. Further research in this area would not only provide valuable insights into the effects of these variables and offer a clearer understanding of the mechanisms driving the obesity paradox but also contribute to the standardization of the sepsis-obesity model.
This study has several limitations. The heterogeneity in experimental designs across the included studies, particularly regarding HFD composition, sepsis induction methods, and animal strains, may have introduced variability that could affect the comparability of results. Although our meta-analysis attempted to standardize some of these variables by focusing on the CLP-DIO model, differences in experimental protocols across studies still make it challenging to draw definitive conclusions. Additionally, the relatively small sample sizes in some of the included studies may have limited the statistical power to detect subtle effects of obesity on sepsis outcomes. Moreover, previous research has indicated that sex may also influence survival in sepsis-obesity models [23]. In this meta-analysis, only one study included female mice, thereby precluding any meaningful subgroup analysis based on sex. Similarly, although the majority of studies used C57BL/6 or C57BL/6J mice, the limited diversity and unequal distribution of strains, including a mixed-strain cohort in one study, prevented reliable analysis of strain-specific effects. Furthermore, the included studies exhibited considerable methodological heterogeneity, not only in the parameters assessed in the meta-regression but also in technical aspects of the CLP procedure, such as needle our meta-analysis found no statistically significant difference in mortality between mice on an HFD and those on a standard diet. These observations indicate that factors beyond obesity per se shape survival in the sepsis-obesity model. Notably, our meta-analysis is the first to quantify how specific time-related variables – age at HFD initiation, HFD duration, and age at sepsis induction – influence survival.
Developing a universally accepted CLP-DIO protocol will require experimental designs that systematically vary key parameters. In addition to the temporal factors identified here, future work must define HFD composition (macronutrient ratios and fatty acid profile), include both sexes and relevant strains, standardize anesthesia, and harmonize CLP technique and adjunct therapies. Moreover, integrating immunometabolic endpoints – comprehensive cytokine and adipokine panels, markers of oxidative stress, metabolic profiling, and microbiome characterization – will link survival outcomes to underlying pathways. A deeper understanding of these factors may help clarify the mechanisms behind the .gauge and the number of punctures (Table 2). While these factors may have influenced the overall severity of peritonitis, systemic inflammation, and survival outcomes across studies, they were consistent within each experiment and therefore did not affect the comparison between obese and lean groups. Taken together, these limitations further highlight the need for standardization in experimental designs to enhance the reproducibility and comparability of findings in the sepsis-obesity model.
Developing a universally accepted CLP-DIO protocol will require dedicated studies that vary key parameters in a factorial manner. In addition to the temporal factors highlighted by our meta-regression (age at HFD initiation, HFD duration, and age at sepsis induction), future optimization should address the composition of the HFD – including macronutrient ratios and a detailed fatty-acid profile – as well as strain and sex of the mice, anesthetic regimen, number of cecal punctures and needle gauge, type and volume of fluid resuscitation, and adjunct therapies such as antibiotics or analgesics. Systematically quantifying the impact of each variable on survival and inflammatory end-points will allow the field to converge on a time-point-standardized, clinically relevant CLP-DIO protocol.
In parallel with standardizing survival protocols, future CLP-DIO studies should incorporate immuno-metabolic endpoints to link phenotypic outcomes with underlying mechanisms and strengthen translational relevance. These endpoints ought to capture systemic inflammation, adipose-tissue signaling, oxidative stress, whole-body metabolic status, and host–microbiome interactions – for example, comprehensive cytokine and adipokine panels, markers of oxidative damage, dynamic glucose–insulin measurements, metabolomic profiling, and characterization of intestinal microbiota.
Although the current meta-analysis was limited to studies using the CLP-DIO model to ensure methodological consistency, this approach inherently restricts generalizability. Other models, such as LPS-induced sepsis in DIO mice, may yield different results; however, substantial variability in experimental design, LPS dosing, and animal characteristics, along with missing data in some reports, precluded a robust meta-analysis of these alternatives. A broader meta-analysis may become feasible in the future as more studies with appropriate methodological details and consistency become available.